November 28, 2011 report
Research group develops more efficient artificial enzyme
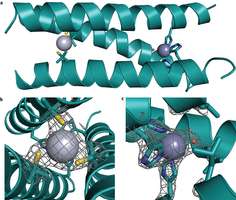
(PhysOrg.com) -- A research group based out of the University of Michigan, and led by Vincent Pecoraro has successfully created a computer designed artificial enzyme that can serve as a catalyst for converting water and carbon dioxide into bicarbonate. In their paper published in Nature Chemistry, the team explains how their metalloprotein (a protein that contains a metal cofactor) mimics carbonic anhydrase and could conceivably lead to a means for removing some of the carbon dioxide in the Earth’s atmosphere that many now believe is contributing to global warming.
Though the artificial enzyme is still many orders of magnitude less efficient than nature’s way of doing things, it is far more efficient than any other artificial process to date, a milestone that gives researchers hope that they will one day equal nature’s abilities.
The mimic, as the team refers to it, is made up of a three-stranded “coiled coil” protein that binds two metals: the active site is zinc, sitting inside the hydrophobic center, while the other site is made up of mercury metals that help to keep the whole works stabilized when introduced to a high pH environment.
The team says that their metalloprotein isn’t necessarily an end product, but more of a way to prove that there likely does exist a path to creating artificial enzymes that can be every bit as efficient as those that occur in nature. The whole point being that if artificial ones can be created, they could be mass produced to exact specifications and used in large scale ways, such as sequestering carbon dioxide in the atmosphere and turning it to a harmless substance that would fall to the ground as bicarbonate.
The next step in the research is to copy the so-called “'second sphere” part of natural enzymes that is missing from the artificial mimic. Its role is to stabilize the differing states during transition and to aid in transferring protons.
It’s because the mimic was able to beat current efficiency models without the second sphere that the researchers are so optimistic about further improvements to their artificial enzyme. Figuring out how to add that second sphere may help the team gain a 100 to 500 fold increase in efficiency of their metalloprotein, which would make it nearly as efficient as Mother Nature, a feat that might yet lead to not only a tool to help combat global warming, but to all manner of applications in the medical field.
More information: Hydrolytic catalysis and structural stabilization in a designed metalloprotein, Nature Chemistry (2011) doi:10.1038/nchem.1201
Abstract
Metal ions are an important part of many natural proteins, providing structural, catalytic and electron transfer functions. Reproducing these functions in a designed protein is the ultimate challenge to our understanding of them. Here, we present an artificial metallohydrolase, which has been shown by X-ray crystallography to contain two different metal ions—a Zn(II) ion, which is important for catalytic activity, and a Hg(II) ion, which provides structural stability. This metallohydrolase displays catalytic activity that compares well with several characteristic reactions of natural enzymes. It catalyses p-nitrophenyl acetate (pNPA) hydrolysis with an efficiency only ~100-fold less than that of human carbonic anhydrase (CA)II and at least 550-fold better than comparable synthetic complexes. Similarly, CO2 hydration occurs with an efficiency within ~500-fold of CAII. Although histidine residues in the absence of Zn(II) exhibit pNPA hydrolysis, miniscule apopeptide activity is observed for CO2 hydration. The kinetic and structural analysis of this first de novo designed hydrolytic metalloenzyme reveals necessary design features for future metalloenzymes containing one or more metals.
Project page: www.umich.edu/~vlpecgrp/resear … /metallopeptide.html
Journal information: Nature Chemistry
© 2011 PhysOrg.com
















