Crystalizing the foundations of better antihistamines
Researchers in Japan have solved the structure of a complex between the antihistamine drug doxepin and its target receptor histamine H1 receptor (H1R)1. Led by So Iwata of Kyoto University and the RIKEN Systems and Structural Biology Center in Yokohama, the team’s findings should aid the development of better treatments for allergies and inflammation.
Histamine, which is released by mast cells of the immune system, is an important mediator of allergic and inflammatory reactions. It exerts its effects by activating cell-surface receptors, thereby triggering cell signaling events. Of the four known human histamine receptor types, H1R is expressed by various tissues, including airways, the vasculature, and the brain.
Pharmacologists have developed various antihistamine drugs that interfere with histamine–receptor interactions. “Many of us will have taken antihistamines to alleviate the symptoms of hay fever, for example, or to stop the swelling and itchiness caused by insect bites,” Iwata says.
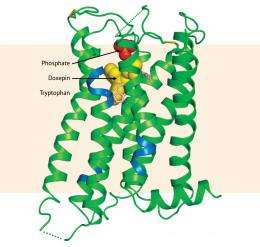
Iwata and his collaborators solved the structure of H1R with bound doxepin using x-ray crystallography. Like all proteins, H1R is composed of amino-acid building blocks. The amino acid tryptophan is found at a particular position in H1R and is known to be important for receptor activation. The researchers revealed that doxepin sits deep within a binding pocket in the receptor, where it interacts directly with this key amino acid (Fig. 1), helping to explain its pharmacological activity.
Doxepin was one of the first antihistamines that effectively blocks histamine receptor activation. Unfortunately, however, these drugs also bind other related receptors. “This low selectivity along with their ability to enter the brain means that these first-generation drugs have considerable side effects such as sedation, mouth dryness, and heart arrhythmias,” explains Iwata.
The researchers’ structural findings suggested that the low selectivity of doxepin is due to the hydrophobic (‘water hating’) nature of the binding pocket, a characteristic found in other receptors to which the drug binds. However, they found that the binding pocket of H1R has a distinctive region occupied by the negatively charged ion phosphate. Through molecular modeling, they demonstrated that the second-generation drugs such as olopatadine would interact with this region, which is not conserved in other related receptors. This explains why these drugs are more selective and have fewer side effects compared with doxepin.
“Our findings demonstrate how minor differences in receptors affect drug selectivity and will be useful in the development of the next generation of antihistamines,” says Iwata.
More information: Shimamura, T., et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 475, 65–70 (2011).
Journal information: Nature
Provided by RIKEN