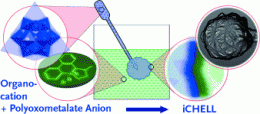
Synthetic cells: Ion exchange leads to complex cell systems with inorganic membranes
(PhysOrg.com) -- Our body consists of individual organs that are made of cells, which in turn contain a number of separate organelles. Biological function cannot be maintained if there are no separate compartments, and compartments are also of use in chemistry. In the journal Angewandte Chemie, a team led by Leroy Cronin at the University of Glasgow (UK) has now introduced a method for the easy production of inorganic chemical cells, known as iCHELLS. Their method even makes it possible to make cells embedded within cells.
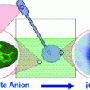
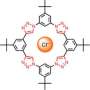
Membranes for synthetic compartments are normally made from high-molecular-weight polymers by aggregation on a surface. In contrast, the iCHELL membranes are made from low-molecular-weight building blocks at the interface of two aqueous solutions. One aqueous solution is simply injected into a second. Solution 1 contains polyoxometallate clusters, tiny “clumps” made of several transition metal atoms, oxygen atoms, and sometimes others. For example, the researchers used a phosphotungstate, a negatively charged cluster in which a phosphorus atom is surrounded by twelve tungsten and 40 oxygen atoms. The counterions are small positively charged ions, such as protons or sodium ions.
Solution 2 contains a compound made of large positively charged organic ions, for example aromatic ring systems, and small negatively charged counterions. When the two solutions come into contact, the ion pairs immediately undergo an exchange of partners: While the two small partners stay in solution, the two large ions come together and aggregate to form a thin membrane because they become insoluble when paired. This forms a cell enclosed by a membrane.
Choosing different ions allows for the thickness and permeability of the membrane to be varied. The membrane can also be given functionality. For example, it is possible to select building blocks that catalyze chemical reactions or recognize specific target molecules. The use of microfluidic systems (chips with tiny fluid-filled channels) makes it possible to easily produce the cells in large numbers, which is a prerequisite for technical applications. Potential uses include encapsulated catalysts in which the membrane would selectively allow the substrate to enter the cell to react.
More complex cell systems can also be made: Simply injecting another solution containing a suitable ion into a cell produces a “cell within a cell”. Such systems could be used as vessels for multistep reactions. However, the biggest goal is the formation of synthetic chemical cells with properties that resemble those of living systems. The scientists hope to gain some indication of how life was able to develop in an inorganic world billions of years ago, and whether it is possible to use the iCHELLS as a platform to develop non-organic “inorganic biology” in the laboratory.
More information: Leroy Cronin, Modular Redox-Active Inorganic Chemical Cells: iCHELLs, Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201105068
Provided by Wiley