A simple compound with surprising antifreeze properties

A chemical compound used to stabilize particles in suspension has proved capable of controlling the growth of ice crystals. This finding was made by CNRS/Saint-Gobain researchers in France. Surprisingly, the compound in question is a simple molecule, not at all like the macromolecules previously known for their antifreeze properties. It offers many advantages, including low production costs, stability and ease of use, which should open the way to industrial applications. Published in the online journal PLoS ONE, this work also provides new leads for the development of synthetic equivalents of antifreeze proteins, different from those currently produced.
The formation of ice crystals can have multiple, and often destructive, consequences. Cell degradation in living organisms, damage to land and roads in cold climates, ice crystals in ice creams… These are all examples of situations where it is useful to control ice growth. Many organisms and species that live in cold environments have adapted to control ice growth. Their resistance to low temperatures is based on the presence of antifreeze proteins, all of which are made up of very long organic chains with amphiphilic structures (partly hydrophilic, partly hydrophobic). How do these proteins interact with ice crystals? Researchers are trying to identify the mechanism enabling antifreeze proteins to identify these crystals, but the phenomenon is still not fully understood. In addition, since these proteins are extremely costly to extract, the preferred solution is to create synthetic equivalents inspired by natural structures. All proteins currently known for their “antifreeze” properties are macromolecules (like glycoproteins, polysaccharides, etc.).
A team led by Sylvain Deville, CNRS researcher at the LSFC (Laboratoire de Synthèse et Fonctionnalisation des Céramiques, Synthesis and Functionalization of Ceramics Laboratory, CNRS/Saint-Gobain), in collaboration with the Matériaux, Ingénierie et Sciences (Materials, Engineering and Sciences) laboratory, has discovered that zirconium acetate, a chemical compound normally used to stabilize particles in suspension, can control ice crystal growth. The compound governs the morphology of the ice crystals obtained by freezing a solution in which it is combined with water. The crystals obtained when adding zirconium acetate are very homogenous, whereas those obtained without it show no particular uniformity.
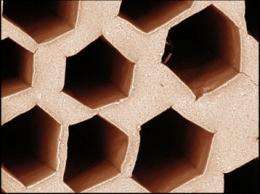
These results are quite surprising, given that zirconium acetate is a “salt,” a simple compound that is radically different from the macromolecules known for their antifreeze properties. It was not known as a substance capable of controlling ice crystal growth. Such control can be exerted in a number of ways: by reducing the speed of crystal growth (to slow their formation), by lowering the freezing point (to delay their formation), or by controlling their morphology, as in this case. Since this implies a direct interaction with the ice crystals, the researchers were surprised to find out that such radically different molecules as zirconium acetate and proteins could affect crystalline growth.
This compound offers significant advantages over existing equivalents, whether natural or synthetic. It is cheap to produce, stable, “simple” and easy to use, which bodes well for a wealth of future industrial applications. In addition, since it is totally different from all previously identified and/or developed substances with the same function, further research could lead to the development of other molecules with antifreeze properties.
This project relied on X-ray diffraction and imaging. These works were made possible by using the X-ray synchrotron (beam line ID19) at the ESRF in Grenoble, France. They are covered by two patents published on October 1, 2011.
More information: Ice shaping properties, similar to that of antifreeze proteins, of a zirconium acetate complex. Sylvain Deville, et al. PLoS ONE. 18 October 2011. doi:10.1371/journal.pone.0026474
Provided by CNRS




















