Study presents approach to access biorelevant structures by 'remodeling' natural products
There is an increasing need for pharmacological tools for biomedical and translational research applications. The field of diversity-oriented synthesis (DOS) has been very fruitful in providing access to numerous new molecules with diverse shapes and chemical structures in order to discover candidate molecules for therapeutic use. Boston University researchers, in a paper published in the journal Nature Chemistry, present a new approach to accessing new, biorelevant structures by "remodelling" natural products. In this case, they demonstrate how the natural product derivative fumagillol can been remodelled to access a collection of new molecules using highly efficient chemical reactions.
"Overall, these studies should pave the way for work to identify pharmacological tools for use in CNS research, oncology, and as anti-infective agents," said John A. Porco, Jr., professor of chemistry at Boston University. "These studies also will enable future studies to remodel additional natural product scaffolds to access novel therapeutic agents."
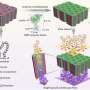
In the search for novel biologically active molecules, DOS strategies break through the limitation of traditional library synthesis by sampling new chemical space. Many natural products can be regarded as useful starting points for DOS, wherein stereochemically rich core structures may be reorganized into chemotypes that are distinctly different from the parent structure. Ideally, to be suited to library applications, such transformations should be general and involve few steps.
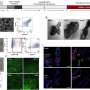
With this objective in mind, Porco and colleagues including Professor John Snyder and postdoctoral fellow Dr. Brad Balthaser successfully remodelled the highly oxygenated natural product fumagillol in several ways using a reaction-discovery-based approach. In reactions with amines, excellent selectivity in a bis-epoxide opening/cyclization sequence was obtained using the appropriate metals catalysts forming either perhydroisoindole or perhydroisoquinoline products. Perhydroisoindoles were further remodelled to other complex structures including novel benzoxazepines.
More information: Nature Chemistry, 23 OCTOBER 2011 DOI: 10.1038/NCHEM.1178
Provided by Boston University Medical Center