Spontaneous combustion in nanobubbles
(PhysOrg.com) -- Nanometer-sized bubbles containing the gases hydrogen and oxygen can apparently combust spontaneously, although nothing happens in larger bubbles. For the first time, researchers at the University of Twente’s MESA+ Institute for Nanotechnology have demonstrated this spontaneous combustion in a publication in Physical Review E. They intend to use the phenomenon to construct a compact ultrasonic loudspeaker.
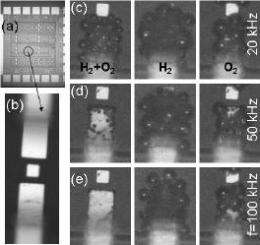
The fact that a violent reaction takes place is already evident from the damage incurred by the electrodes with which the reaction is initiated. These electrodes are used to make hydrogen and oxygen by electrolysis, in the usual manner, in an ultra-small reaction chamber. If the plus and minus poles are continually alternated, tiny bubbles containing both gases arise.
The frequency with which the poles are alternated determines the size of the bubbles: the higher the frequency, the smaller the bubbles. Combustion only takes place in bubbles that are smaller than 150 nanometres (a nanometre is a millionth of a millimetre); nothing happens in larger bubbles. Early experiments in microreactors also showed that nothing happened in larger bubbles; the heat can dissipate to the larger internal surface.
Meters per second
Researcher Vitaly Svetovoy was working on the construction of an actuator for rapidly building pressure when he came across this phenomenon. Such actuators are, for example, used in loudspeakers for ultrasonic frequencies undetectable by the human ear in the medical world. None of the mechanical techniques currently available are suitable for making a very compact loudspeaker of this kind and still achieving a 'deflection' of metres per second on this scale. Svetovoy thought, however, that it might be possible by building up pressure with bubbles. The problem was that the bubbles could be made very rapidly but that they did not disappear quickly enough. The combustion reaction that has now been demonstrated might solve this problem. But it causes other problems too, such as the damage to the electrodes. "That is what we now have to look at", Svetovoy said.
This research was carried out by Prof. Miko Elwenspoek's Transducer Science and Technology group of the University of Twente's MESA+ Institute for Nanotechnology.
The article 'Combustion of hydrogen-oxygen mixture in electrochemically generated nanobubbles' by Vitaly Svetovoy, Remko Sanders, Theo Lammerink and Miko Elwenspoek appeared in Physical Review E on 23 September 2011.
Provided by University of Twente