Study finds protein critical to breast cancer cell proliferation, migration
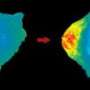
Researchers have found that a protein linked to cell division and migration and tied to increased cell proliferation in ovarian tumors is also present at high levels in breast cancer specimens and cell lines. The protein, dubbed "UNC-45A," was also determined to be more active in breast cancer cells than in normal breast cells.
University of Texas Medical Branch at Galveston scientists describe these findings and others in a paper now online in the Journal of Molecular Biology.
"As a result of earlier work, we hypothesized that UNC-45A should be critical in several steps related to cancer cell metastasis," said UTMB professor Henry Epstein. "This investigation confirmed that hypothesis, and also showed us significant aspects of UNC-45A's behavior that were previously unknown."
UNC-45A is what is known as a "chaperone" protein, a molecule that helps other proteins function more effectively. In the case of UNC-45A, the protein is myosin, which can be thought of as a tiny machine that interacts with a long, fiber-like protein called actin to alter cell shape and movement. In the last stage of cell division, for example, myosin and actin proteins pinch the cell tightly about its midsection, finally splitting a single cell into two daughter cells.
"What we believe is really important in this paper is that increased UNC-45A in cancer cells leads to enhanced myosin and actin activity, which leads to increased rates of cell proliferation and increased rates of cancer-cell invasion or migration," Epstein said. "Those are critical phenomena and could be significant in the development of new therapeutic approaches."
Epstein's group measured UNC-45A's effect on myosin and actin activity by comparing the activity observed in cells from a highly metastatic cancer cell line with that seen in cells from the same line in which UNC-45A production had been blocked. The difference was substantial, strongly suggesting that high levels of UNC-45A drove the cells' high rate of proliferation and invasion of other tissues.
Further exploring the details of UNC-45A, the UTMB team discovered that the protein actually exists in two slightly different isoforms, one made up of 944 amino acids and the other of 929 amino acids. While these two isoforms interacted similarly with myosin, the breast cells' protein breakdown apparatus attacked the 944 amino acid-isoform much more vigorously than the 929 amino-acid isoform; as a result, the 929 amino-acid isoform was found in much greater levels.
"In the breast cancer cells, you get a disregulation of the two, because the larger one gets turned over more rapidly than the smaller one, and we can actually see this very dramatically," Epstein said.
Provided by University of Texas Medical Branch at Galveston