Key protein reveals secret of stem cell pluripotency
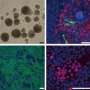
A protein that helps maintain mouse stem cell pluripotency has been identified by researchers at the RIKEN Omics Science Center. The finding, published in the August issue of Stem Cells (first published online July 26, 2011), points the way to advances in regenerative medicine and more effective culturing techniques for human pluripotent stem cells.
Through their capacity to differentiate into any other type of cell, embryonic stem cells (ES cells) and induced-pluripotent stem cells (iPS cells) promise a new era of cell-based treatments for a wide range of conditions and diseases. Cultivating such cells, however, commonly relies on the use of so-called “feeder” cells to maintain pluripotency in cell culture conditions. Feeder cells keep stem cells in their undifferentiated state by releasing nutrients into the culture medium, but they have the potential to introduce contamination which, in humans, can lead to serious health risks.
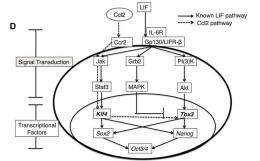
Previous research has shown that mouse pluripotent stem cells can be cultured without feeder cells through the addition of a cytokine called Leukemia Inhibitory Factor (LIF) to the culture media (“feeder-free” culture). LIF is secreted by mouse feeder cells and activates signal pathways reinforcing a stem cell regulatory network. The researchers discovered early in their investigation, however, that the amount of LIF secreted from feeder cells is much less than the amount needed to maintain pluripotency in feeder-free conditions. This points to other, as-of-yet unknown contributing factors.
To clarify these factors, the research group analyzed differences in gene expression between mouse iPS cells cultured on feeder cells and those cultured in feeder-free (LIF treated) conditions. Their results revealed 17 genes whose expression level is higher in feeder conditions. To test for possible effects on pluripotency, they then selected 7 chemokines (small proteins secreted by cells) from among these candidates and overexpressed them in iPS cells grown in feeder-free conditions. They found that one chemokine in particular, CC chemokine ligand 2 (CCL2), enhances the expression of key pluripotent genes via activation of a well-known signal pathway known as Jak/Stat3.
While CCL2 is known for its role in recruiting certain cells to sites of infection or inflammation, the current research is the first to demonstrate that it also helps maintain iPS cell pluripotency. The findings also offer broader insights applicable to the cultivation of human iPS/ES cells, setting the groundwork for advances in regenerative medicine.
More information: Yuki Hasegawa, et al. "CC Chemokine Ligand 2 and Leukemia Inhibitory Factor Cooperatively Promote Pluripotency in Mouse Induced Pluripotent Cells." Stem Cells, 2011, DOI: 10.1002/stem.673
Provided by RIKEN