March on, Hydrogen! Mild but very efficient: new catalytic process extracts hydrogen from bioalcohols
(PhysOrg.com) -- Over 80% of the worlds energy demands continue to be met with fossil fuels. The environmental problems associated with this, such as global warming, are well-known. The efficient supply of energy based on renewable resources is becoming more pressing. Hydrogen technology, which involves the production of hydrogen from biomass for use in electricity production in fuel cells, is a very promising approach.
In the journal Angewandte Chemie, researchers led by Matthias Beller at the Leibniz Institute for Catalysis in Rostock (Germany) have now introduced a new catalyst that allows for the use of bioalcohols for the production of hydrogen. Their novel process proceeds efficiently under particularly mild conditions.
Ethanol and other alcohols do not willingly give up their hydrogen atoms; this type of reaction requires highly active catalysts. Previous catalytic processes require downright drastic reaction conditions: temperatures above 200 C and the presence of strong bases. The Rostock researchers thus aimed to develop a catalyst that would also work efficiently at significantly milder temperatures.
Martin Nielson, working on Beller’s team thanks to an Alexander von Humboldt scholarship, has now been successful. The new catalyst demonstrates previously unachievable high efficiency in the extraction of hydrogen from alcohols under mild reaction conditions. Says Beller, “This is the first catalytic system that is capable of obtaining hydrogen from readily available ethanol at temperatures under 100 C without the use of bases or other additives.”
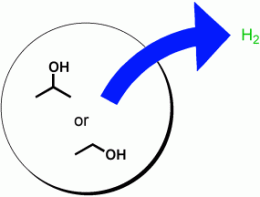
After initial successful tests with a relatively easily converted model alcohol (isopropanol), the researchers turned their attention to ethanol, also known as the “alcohol” in alcoholic beverages. Ethanol has taken on increasing importance as a renewable resource but is significantly harder to convert. “Even with ethanol, this new catalyst system demonstrated an unusually good conversion rate under milder conditions (60–80 C),“ says Beller. “In comparison to previous catalyst systems, this one is nearly an order of magnitude higher.”
The active catalyst consists of a ruthenium complex that is formed in situ. The starting point is a central ruthenium atom that is surrounded by a special ligand that grasps it from three sides. The other ligands are a carbon monoxide molecule and two hydrogen atoms. Upon heating, a hydrogen molecule (H2) is released from the complex. When the remaining complex comes into contact with ethanol or isopropanol it grabs two replacement hydrogen atoms, allowing the cycle to begin again.
More information: Matthias Beller, Efficient Hydrogen Production from Alcohols under Mild Reaction Conditions, Angewandte Chemie International Edition 2011, 50, No. 41, 9593–9597, dx.doi.org/10.1002/anie.201104722
Provided by Wiley