Hydrogen released to fuel cell more quickly when stored in metal nanoparticles
Researchers from TU Delft and VU University Amsterdam in the Netherlands have demonstrated that the size of a metal alloy nanoparticle influences the speed with which hydrogen gas is released when stored in a metal hydride. The smaller the size of the nanoparticle, the greater the speed at which the hydrogen gas makes its way to the fuel cell. The researchers publish their findings in the October issue of the scientific journal Advanced Energy Materials.
On 27 September Dutch Minister of Infrastructure and the Environment, Ms Schultz van Haegen, announced she will earmark 5 million Euros to stimulate hydrogen transport in the Netherlands. According to the Minister the Netherlands and neighbouring countries have all it takes to become a 'hydrogen heaven'. In July 2011, the German car manufacturer Daimler announced its intention to build twenty new hydrogen fuelling stations along Germany's motorways. Hydrogen is back on the agenda. Hydrogen gas is currently stored in a vehicle fuel tank at 700 bar pressure. Fuelling stations thus require high-pressure pumps to fill these tanks and these systems consume a lot of energy.
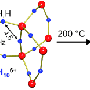
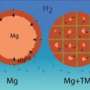
There are thus good reasons for finding alternative hydrogen storage techniques. Hydrogen can be absorbed in high densities in metals such as magnesium, without the need for high pressure. However, the disadvantage is that releasing the hydrogen again is a very difficult and very slow process. One way of speeding up the release of the hydrogen is to use magnesium nanoparticles that are fixed in a matrix to prevent them from aggregating.
Professor of Materials for Energy Conversion and Storage, Bernard Dam, and his colleagues at TU Delft and VU University Amsterdam have demonstrated experimentally that the interaction between the nanoparticles and the matrix can cause the hydrogen gas to be released faster. Using models consisting of thin layers of magnesium and titanium, they show how the pressure of the hydrogen being released from the magnesium increases as the layers become thinner. This means that it indeed makes sense to store hydrogen in nanoparticles in a matrix. The choice of matrix determines to what extent the hydrogen desorption pressure increases. The researchers published their findings in the October 2011 edition of the scientific journal Advanced Energy Materials.
Efficient and affordable hydrogen storage techniques can play an important role in the large-scale adoption of hydrogen fuel cells. Bernard Dam foresees the development of hybrid vehicles that use batteries for short distances but switch to hydrogen for long distances: 'Your electric motor will be powered by batteries inside the city, and by hydrogen when you go further afield.'
Provided by Delft University of Technology