September 15, 2011 feature
Graphene nanoribbons grow due to domino-like effect
(PhysOrg.com) -- While many labs are trying to efficiently synthesize large two-dimensional sheets of graphene, a team of researchers from Sweden and the UK is investigating the synthesis of very thin strips of graphene just a few atoms wide. In contrast to graphene, these graphene nanoribbons have a unique electronic structure including a non-zero band gap, which makes them promising candidates for semiconductor applications. But, as with graphene sheets, one of the greatest challenges for now is finding a way to efficiently synthesize these graphene nanoribbons.
In their study, researchers Jonas Björk and Sven Stafström from Linköping University in Sweden and Felix Hanke from the University of Liverpool in the UK have used a powerful supercomputer at Linköping University to investigate how graphene nanoribbons grow from an anthracene polymer on a gold substrate. The results of their study are published in a recent issue of the Journal of the American Chemical Society.
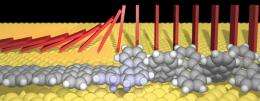
The scientists discovered that, in the most likely nanoribbon growth process, the gold substrate acts as more than just a support where the reaction can take place. The gold actually catalyzes the reaction by attracting hydrogen atoms from the anthracene polymer (which is made of benzene rings) to bind to the gold surface, initiating the first step of the reaction. In this “dehydrogenation” process, two hydrogen atoms from each unit of the anthracene polymer are transferred to the gold surface, leaving behind a carbon-carbon bond. The carbon-carbon bond forms part of graphene’s honeycomb lattice. Meanwhile, the hydrogen atoms are released from the gold surface through desorption into the vacuum.
The supercomputer also revealed that this dehydrogenation reaction repeats due to the effects of positive cooperativity: When a polymer unit has a neighbor that has a carbon-carbon bond, its probability for undergoing the same reaction and gaining its own carbon-carbon bond increases. The result is that the reaction, which starts at one end of the polymer, propagates unit by unit through the entire polymer in a domino-like fashion. After several minutes, the entire polymer is transformed into a well-defined graphene nanoribbon with a width of seven carbon atoms.
Figuring out how graphene nanoribbons are synthesized in this way is a complicated molecular-scale process that can only be unraveled in detail by powerful supercomputers. Although there are a few other reaction pathways that the reaction could take, the researchers calculated that this reaction is very highly favored over the others: They estimated that 10,000 reactions proceed along this route than by the second most favorable reaction. Understanding the reaction will allow the researchers to identify the best fabrication method for future experiments and development.
“This is a question about how to build materials, either 'bottom-up' (synthesis from its constituents) vs. 'top-down' (taking something bigger and cutting it to size),” Hanke told PhysOrg.com. “The bottom-up in the graphene nanoribbons approach is very interesting as it allows us to start with the ultimate size limit for a material (an atom, or, say, a small molecule) and then add only those pieces that are really, really needed. Moreover, it also allows us to make graphene nanoribbons that have consistently the same width of, say, seven Angstroms (7x10-10 m), simply by making sure that the ingredients are only polyanthracene and not anything much bigger. This sounds trivial, but it is actually very difficult to achieve in top-down approaches, particularly if atomic precision is desired.”
The applications of graphene nanoribbons (and graphene itself) are still in the very early stages, but their properties make the materials look promising. Previous studies have shown that controlling the widths and edge structures of graphene nanoribbons can tune the ribbons’ electronic properties, which could lead to molecular-based electronics such as transistors. By gaining a better understanding of how graphene nanoribbons grow, including the catalytic role of the gold substrate and domino effect of the reaction, scientists have taken another step toward this future technology.
“The main hype behind graphene nanoribbons is that you should be able to use them for semiconductor applications, which is due to their highly desirable electronic structure that is different from the electronic structure of graphene,” Hanke said. “The beauty of graphene nanoribbons is that their electronic response is determined simply by their shape. Therefore, being able to understand and build graphene nanoribbons in a controlled manner is a very important process for the continued development of electronics. In particular for anthracene-based nanoribbons, we have a width that is still about 30 times smaller than what is available in current semiconductor-based electronics.”
More information: Jonas Björk, et al. “Zipping Up: Cooperativity Drives the Synthesis of Graphene Nanoribbons.” Journal of the American Chemical Society. DOI:10.1021/ja205857a
Copyright 2011 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.