A 'jumping gene's' preferred targets may influence genome evolution
The human genome shares several peculiarities with the DNA of just about every other plant and animal. Our genetic blueprint contains numerous entities known as transposons, or "jumping genes," which have the ability to move from place to place on the chromosomes within a cell.
An astounding 50% of human DNA comprises both active transposon elements and the decaying remains of former transposons that were active thousands to millions of years ago before becoming damaged and immobile. If all of this mobile and formerly mobile DNA were not mysterious enough, every time a plant, animal or human cell prepares to divide, the chromosome regions richest in transposon-derived sequences, even elements long deceased, are among the last to duplicate. The reason for their delayed duplication, if there is one, has eluded biologists for more than 50 years.
New research led by Carnegie's Allan Spradling and published online this week by Proceedings of the National Academy of Sciences provides potential insight into both these enigmas.
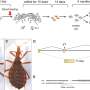
The scientists used the fruit fly, Drosophila melanogaster, one of the premier "model" organisms for studying genome structure and gene function. They focused on one particular transposon, called the P element, which has an unsurpassed ability to move that has stimulated its widespread use by Drosophila researchers.
Remarkably, P elements have only been present in Drosophila melanogaster for about 80 years, at which time they were acquired from the genome of a distantly related fruit fly species by an unknown process. P elements remain highly "infective" today. Adding just one copy to the genome of one fly causes all the flies in a laboratory population with which it breeds to acquire 30 to 50 P elements within a few generations. The original goal of the Spradling team's research was not to understand how transposons spread or genomes evolve, but something much simpler: To learn why P elements insert at some locations in the genome but not in others.
Spradling and his colleagues, who oversee the NIH-funded Drosophila "Gene Disruption Project" used a database containing more than 50,000 genomic sites where P elements have inserted. They built this exceptional database over the last 20 years.
P elements insert into DNA very selectively. Nearly 40% of new jumps occur within just 300 genes and always near the beginning of the gene. But the genes seemed to have nothing in common. When these sites were compared to data about the Drosophila genome, particularly recent studies of Drosophila genome duplication, the answer became clear. What many P insertion sites share in common is an ability to function as starting sites or "origins" for DNA duplication. This association between P elements and the machinery of genome duplication suggested that they can coordinate their movement with DNA replication.
Spradling and his team propose that P elements—and likely other transposons as well—use a replication connection to spread more rapidly through genomes. These elements would only transpose after replicating, and then preferentially insert themselves into portions of DNA that have not yet become activated. This would allow them to duplicate twice rather than just once during the genome duplication cycle.
If the elements get a late start, however, only the last segments of the chromosome to duplicate will be left for their second duplication. This explains tendency of such regions to be transposon-rich. However, the researchers found that two other Drosophila transposons, known as piggyBac and Minos, do not insert at replication origins, so this mechanism is far from universal. Furthermore, Spradling cautioned that it is particularly difficult to experimentally test hypotheses about evolution.
"By gaining insight into one specific transposon's movements, we may have begun to glimpse mechanisms that have profoundly influenced genome evolution for nearly all animals" Spradling commented.
Provided by Carnegie Institution