Carbon nanotube structures changed by 'attack' from within, researchers discover
(PhysOrg.com) -- A team of researchers involving scientists from The University of Nottingham has shown for the first time that chemical reactions at the nano-level which change the structure of carbon nanotubes can be sparked by an 'attack' from within.
The discovery challenges previous scientific thinking that the internal surface of the hollow nanostructures is chemically unreactive, largely restricting their use to that of an inert container or a ‘nano-reactor’ inside which other chemical reactions can take place.
Their research, published in the journal Nature Chemistry, shows that carbon nanotubes that have had their structures changed are exciting new materials that could be useful in the development of new technologies for gas storage devices, chemical sensors and parts of electronic devices such as transistors.
Dr Andrei Khlobystov, of the University’s School of Chemistry, who led the work at Nottingham, said: “It has universally been accepted for some time now that the internal surface of carbon nanotubes — or the concave side — is chemically unreactive, and indeed we have been successfully using carbon nanotubes as nano-reactors.
“However, in the course of this new research we made the serendipitous discovery that in the presence of catalytically active transition metals inside the nanotube cavity, the nanotube itself can be involved in unexpected chemical reactions.”
Carbon nanotubes are remarkable nanostructures with a typical diameter of 1–2 nanometres, which is 80,000 times smaller than the thickness of a human hair. Dr Khlobystov and his research associates were recently involved in the discovery — published in Nature Materials — that nanotubes can be used as a catalyst for the production of nanoribbon, atomically thin strips of carbon created from carbon and sulphur atoms. These nanoribbons could potentially be used as new materials for the next generation of computers and data storage devices that are faster, smaller and more powerful.
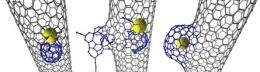
In this latest research, the scientists found that an individual atom of Rhenium metal (Re) sets off a chemical reaction leading to the transformation of the inner wall of the nanotube. Initially, the attack by the Rhenium creates a small defect in the nanotube wall which then gradually develops into a nano-sized protrusion by ‘eating’ additional carbon atoms.
The protrusion then rapidly increases in size and seals itself off, forming a unique carbon structure dubbed a NanoBud, so called because the protrusion on the carbon nanotube resembles a bud on a stem.
Previously, NanoBuds were believed to be formed outside the nanotube through reactions on the outer surface with carbon molecules called fullerenes.
The new study demonstrates for the first time that they can be formed from within, provided that a transition metal atom with suitable catalytic activity is present within the nanotube.
In collaboration with the Electron Microscopy of Materials Science group at Ulm University in Germany, the scientists have even been able to capture ‘on camera’ the chemical reaction of the transition metal atom with the nanotube in real time at the atomic level using the latest Aberration-Corrected High Resolution Transmission Electron Microscopy (AC-HRTEM). Their videos show nanotubes with a diameter of around 1.5 nanometers, while the NanoBuds are just 1 nanometer across.
Provided by University of Nottingham

















