New scientific research reveals diamonds aren't forever

(PhysOrg.com) -- In a paper published in the US journal Optical Materials Express this week, Macquarie University researchers show that even the earth's hardest naturally occurring material, the diamond, is not forever.
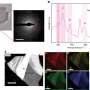
Associate Professor Richard Mildren and his colleagues from the Macquarie University Photonics Research Centre discovered that diamonds evaporate under exposure to light.
"Although this type of light-induced evaporation has been observed in some materials, this is the first time it's been shown to occur for diamond," Mildren said.
The diamonds were exposed to intense light pulses in the UV-C band (the harsh ultraviolet rays filtered out by the ozone layer), and small pits in the diamond surface were visible after only a few seconds. The rate of mass loss in the diamond fell notably for lower light levels but the etching process still continued - albeit at a slower and slower pace, Mildren said.
But before diamond lovers around the world start to panic, he is quick to note that the rate of evaporation is very small and not noticeable under normal conditions. In fact, even under very bright UV conditions, such as intense sunlight or under a UV tanning lamp, it would take approximately the age of the universe - about 10 billion years - to see an observable distance, he said.
The findings not only provide clues about the long-term stability of diamonds, but also have broad implications for future research.
"It's a very practical discovery and we are now looking at how we can exploit this," Mildren said.
"If we can make structures in the diamonds that enable us to control the position of the light within a very narrow filament in the diamond, that's the first step to making smaller and more efficient optical devices such as those used in quantum computing and high performance lasers."
The discovery may also have implications as far reaching as the prospects for finding diamonds on the surface of other planets, Mildren said.
More information: Mildren, R. P. et al. Opt. Mater. Express 1, 576-585 (2011).
Provided by Macquarie University