Embracing superficial imperfections
Chemists normally work rigorously to exclude impurities from their reactions. This is especially true for scanning tunneling microscopy (STM) experiments that can produce atomic-scale images of surfaces. Using STM to investigate processes such as catalysis usually requires pristine substrates—any flaws or foreign particles in the surface can critically interfere with the test study. Preconceptions about interface defects and catalysis are about to change, however, thanks to recently published research led by Yousoo Kim and Maki Kawai at the RIKEN Advanced Science Institute in Wako, Japan.
Through a series of high-level computer simulations, the researchers found that the catalytic splitting of water molecules occurs faster on an ultrathin insulating film containing misplaced atoms than on a non-defective surface. Because water splitting reactions are one of the easiest ways to generate hydrogen fuel, this finding could be a boon to future fleets of hybrid vehicles.
Recently, Kim, Kawai, and colleagues discovered that depositing insulating magnesium oxide (MgO) onto a silver (Ag) substrate enabled extraordinary control over water dissociation reactions. By injecting electrons into the MgO/Ag surface with an STM tip, they were able to excite absorbed water molecules and cause them to sever hydrogen and hydroxide ions. Optimizing the MgO film thickness was a key part of this approach; only ultrathin layers could direct water splitting owing to its enhanced electronic interaction strength.
This relationship between insulator thickness and chemical reactivity suggested to the researchers that the oxide–metal interface plays a crucial role in directing catalytic reactions. Engineering specific flaws into the ultrathin interface could be one way to heighten the electronic control over the water-splitting process. However, since artificially manipulating oxide atoms is a difficult experimental procedure, they used density functional theory simulations, based on quantum mechanics, to analyze the role of structural imperfections in MgO.
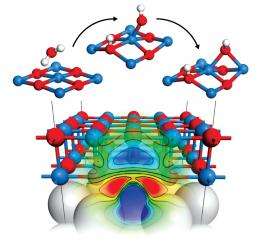
Surprisingly, the researchers found that three different types of defects—oxygen and magnesium impurities, as well as an oxygen vacancy—improved water adsorption and substantially lowered dissociation energy barriers compared to an ideal MgO film. Further analysis revealed that the oxide defects accumulate charges injected into the substrate (Fig. 1), creating an electronic environment that speeds up the catalytic water splitting. “In the presence of these defects, the film’s chemical reactivity can be greatly enhanced,” says Kim.
The next goal for the researchers is to find systematic techniques to control interface imperfections on these novel catalytic films—an objective best achieved by the team’s unique combined experimental–theoretical approach, notes Kim.
More information:
1. Jung, J., et al. Activation of ultrathin oxide films for chemical reaction by interface defects. Journal of the American Chemical Society 133, 6142–6145 (2011).
2. Shin, H.-J., et al. State-selective dissociation of a single water molecule on an ultrathin MgO film. Nature Materials 9, 442–447 (2010).
3. Jung, J., et al. Controlling water dissociation on an ultrathin MgO film by tuning film thickness. Physical Review B 82, 085413 (2010).
Provided by RIKEN




















