New finding shows a research area to expand in EMSL Radiochemistry Annex
Scientists from Pacific Northwest National Laboratory and Rai Enviro-Chem, LLC, recently published first-ever results that illustrate the importance of determining hard-to-find oxidized Fe(III) reaction products in the reduction of Pu(IV) to Pu(III) by the reductant Fe(II). The shift from the insoluble Pu(IV)—the current state of plutonium contaminants within sediments at the Department of Energy’s Hanford Site—to the lower oxidation state Pu(III) is a very important reaction to study because Pu(III) is soluble, and therefore potentially more mobile in the groundwater.
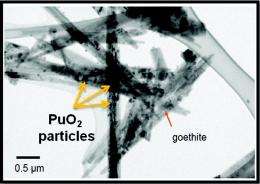
However, this particular reduction reaction is far less studied than other contaminant-related reactions because of the radioactivity of the samples and the need for specialized facilities and equipment. The research team’s overall strategy was to move beyond bulk studies of Pu(IV) /Fe(II) interactions to explore the microscopic and molecular processes involved in forming the tiny amounts of Fe(III) oxidization products that the reaction generates—a challenge that had never been attempted.
In this case, the team coupled solution-phase measurements of Pu concentrations and oxidation state determinations with SEM/TEM analysis of the reaction products, which demonstrated the enhancing role that Fe(III) reaction products play in the formation of Pu(III), and led to development of the first thermodynamic theory of such an effect. This outcome is a considerable step toward understanding the molecular mechanisms that govern the reduction from Pu(IV) to Pu(III), while also arming engineers with new information on how to inhibit the reaction in contaminated subsurface environments around the nation. Studies like these will be greatly enhanced by the addition of EMSL’s new Radiochemistry Annex, which is set to fully open to the global user community in Fall 2012. Until then, selected new radiological capabilities will become available to users beginning in August 2011.
More information: Felmy AR, et al. 2011. "Heterogeneous Reduction of PuO2 with Fe(II): Importance of the Fe(III) Reaction Product." Environmental Science & Technology 45:3952-3958. DOI: 10.1021/es104212g
Provided by Environmental Molecular Sciences Laboratory