June 7, 2011 report
After three years of review, two new elements finally added to the Periodic Table
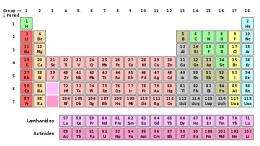
(PhysOrg.com) -- After three years of review, a committee representing the governing bodies of both chemistry and physics, has published a paper on Pure and Applied Chemistry, accepting the work of a collaborative team of physicists as proof of the creation of element 114 and element 116, finally allowing them both to be added to the official Periodic Table of Elements.
The two as yet unnamed new elements, currently going by ununquadium and ununhexium, are now the two heaviest elements on the table (289 and 292 atomic mass, respectively) and both are highly radioactive.
114 had several groups which claimed to have produced it in a lab, but just two teams had sufficient evidence for the examining committee to give approval; the Joint Institute for Nuclear Research (JINR) in Dubna, Russia and Lawrence Livermore National Laboratory in California. The two groups collaborated on the project and both groups were also credited with offering proof of the existence of 116 as well.
The new elements came about as the result of hurtling lighter atoms together in an accelerator. To forge 116, they threw curium (96 protons) and calcium (20 protons) together, which shortly thereafter decayed to 114. But they also made 114 by knocking calcium and plutonium together.
Properties of the new elements, such as how they might react with other elements, have yet to be discovered however, as both last less than a second before decaying away to other elements.
Both new elements have actually been known to exist for quite some time; as far back as 1999 different groups were said to have produced it in a lab, and most of the work done by the collaborating teams was done back in 2004 and 2006.
The two latest additions to the periodic table ignite new blather about the possible existence of a so-called “island of stability” where chemists and physicists debate the possibility of much heavier elements eventually joining the table; ones that will be extremely stable, and thus ripe for use right out of the box.
Traditionally the folks that produce a new element are the ones that get to name it, so for 114 and 116, that will be the next step; likely something Russian, since the Russian team gets most of the credit, and as far as the approval committee goes, it’s likely to be a smooth process, so long as, according to one committee member, “it’s not something too weird.”
More information: IUPAC announcement: www.iupac.org/web/nt/2011-06-01_elements_114_116
Discovery of the elements with atomic numbers greater than or equal to 113 (IUPAC Technical Report), Pure Appl. Chem., ASAP article, doi:10.1351/PAC-REP-10-05-01
Abstract
The IUPAC/IUPAP Joint Working Party (JWP) on the priority of claims to the discovery of new elements 113–116 and 118 has reviewed the relevant literature pertaining to several claims. In accordance with the criteria for the discovery of elements previously established by the 1992 IUPAC/IUPAP Transfermium Working Group (TWG), and reinforced in subsequent IUPAC/IUPAP JWP discussions, it was determined that the Dubna-Livermore collaborations share in the fulfillment of those criteria both for elements Z = 114 and 116. A synopsis of experiments and related efforts is presented.
© 2010 PhysOrg.com