Membrane pockets that gain catalytic activity upon self-assembly shed light on biological enzymatic processes
Biological membranes play key roles in the body. They determine, for example, how molecules enter and exit cells, and the architecture of their lipid bilayer allows them to host enzymes and enhance their catalytic performance under natural conditions. To clarify the mechanisms that govern these processes, a team of chemists in Japan has generated in water tiny, catalytically active, free-standing membrane pockets, called vesicles, using a self-assembly method based on a small palladium complex. The team was led by Yasuhiro Uozumi from the RIKEN Advanced Science Institute in Wako and the Institute for Molecular Science in Okazaki.
Many researchers have already used ultra-small self-assembled pockets to perform reactions in solution while protecting the reagents from their potentially destructive surroundings. However, unlike Uozumi’s vesicles, few of these reaction vessels were ‘architecture-based’ catalysts; that is, structures that exhibit activity only when self-assembled.
The team’s palladium complex is a rigid, planar, pincer-like structure with hydrophilic ‘arms’ and hydrophobic ‘legs’. The different affinity for water and orientation of these functional groups directs vesicle assembly in water. Furthermore, these properties allow the complex to gain unique catalytic activity for specific chemical reactions. “This, conceivably, would approach an artificial enzymatic system,” notes Uozumi.
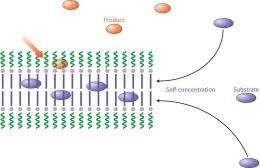
“The vesicle, which bears a hydrophobic inner region, was self-constructed in water, and this inner region served as a reservoir for the substrate,” says Uozumi. He explains that the entire reaction system—including the medium, molecular structure of the palladium complex, and substrate—cooperatively governs a ‘self-concentration’ process. During this process, substrate molecules penetrate the hydrophilic outer shell and accumulate in the hydrophobic reservoir where the reaction takes place (Fig. 1). After a quick catalytic transformation, the product exits the vesicle.
The researchers conducted a series of carbon–carbon bond-forming reactions, which are central to chemical synthesis, in the presence of the vesicles. They found that the vesicles stimulated the transformations in high yields at room temperature in water. The palladium complex was also recoverable in its original, disassembled form after the reaction. When they ran the same experiment in hydrophobic organic solvents, which hinder vesicle formation, no catalysis occurred—proof that water-mediated self-assembly is crucial for the catalytic activity of the complex.
The team is currently developing new catalysts by changing the hydrophilic and hydrophobic groups and substituting palladium for other metal species. It is also applying these catalysts to other organic reactions. These water-enabled transformations will lead to greener and safer approaches to organic chemistry, Uozumi concludes.
More information: Hamasaka, G., et al. Molecular-architecture-based administration of catalysis in water: self-assembly of an amphiphilic palladium pincer complex. Angewandte Chemie, International Edition 50, 4876–4878 (2011).
Provided by RIKEN