Citrate key in bone's nanostructure
Bone is one of nature's surprising "building materials." Pound-for-pound it's stronger than steel, tough yet resilient. Scientists at the U.S. Department of Energy's Ames Laboratory have identified the composition that gives bone its outstanding properties and the important role citrate plays, work that may help science better understand and treat or prevent bone diseases such as osteoporosis.
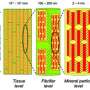
Using nuclear magnetic resonance (NMR) spectroscopy, Ames Laboratory scientist and Iowa State University chemistry professor Klaus Schmidt-Rohr and his colleagues studied bone, an organic-inorganic nanocomposite whose stiffness is provided by thin nanocrystals of carbonated apatite, a calcium phosphate, imbedded in an organic matrix of mostly collagen, a fibrous protein.
By understanding the nanostructure of naturally occurring materials, researchers may be able to develop new light-weight, high-strength materials that will require less energy to manufacture and that could make the products in which they are used more energy efficient.
"The organic, collagen matrix is what makes bones tough," Schmidt-Rohr said, "while the inorganic apatite nanocrystals provide the stiffness. And the small thickness – about 3 nanometers – of these nanocrystals appears to provide favorable mechanical properties, primarily in prevention of crack propagation."
While bone structure has been studied extensively, how these apatite nanocrystals form and what prevents them from growing thicker was a mystery. Some research pointed to sugars being involved, but that didn't match with the NMR spectra that Schmidt-Rohr was seeing.
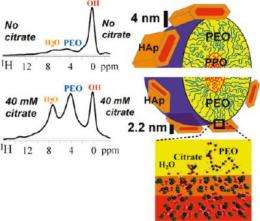
"We can see all the peaks clearly," he says of a spectral graph which shows the points at which specific components in bone samples resonate; these specific signatures are the key to NMR technology, "even those at the organic-inorganic interface, where the organic material's signal strength is relatively weak."
After studying bone structure over a five-year period, it was actually serendipitous that Schmidt-Rohr came across a signature that appeared to match what he was seeing.
"We had gotten some crystalline collagen samples to study," he said, "and it turned out that the supplier, Sigma-Aldrich, had used citrate to dissolve the collagen. And the citrate signature in the collagen samples matched the signature we were seeing in bone."
According to Schmidt-Rohr, the role of citrate in bone had been studied up until about 1975, but since that time, no mention was made in any of the newer literature on bone. So in essence, his research team had to rediscover it.

The case for citrate was made most convincingly when graduate research assistant Yanyan Hu was able to extract citrate from cow bone and replace it with carbon 13 (C13) -enriched citrate, resulting in a 30-fold enhancement of the NMR signals of the bone sample. The peaks matched exactly, confirming the presence of citrate on the surface where the apatite nanocrystals had formed.
Schmidt-Rohr further hypothesized that, since citrate is too large to be incorporated into the apatite crystal lattice, it must be bound to the nanocrystals' surface where it stabilizes the nanocrystals' size by preventing their further growth. The findings were published in the Dec. 28, 2010 issue of the Proceedings of the National Academy of Sciences.
"Based on the old literature, we looked at the citrate levels in a variety of types of bone and found that herring spine had the highest citrate concentration – about 13 percent by weight," Schmidt-Rohr said. "So it should hold that the citrate signal for herring spine should be three times higher than for cow bone, and indeed it was."
In further studies, the group found that higher concentration of citrate, the thinner the apatite nanocrystals in bone. This was further confirmed on bone-mimetic nanocomposites in a collaboration with Ames Lab faculty scientists Surya Mallapragada and Muffit Akinc, using a polymer template with various concentrations of citrate to synthesize apatite nanocrystals. At higher concentrations, the nanocrystals that formed were thinner and should therefore be more resistant to crack propagation. This work was published in the April 12 issue of Chemistry of Materials.
"At this point, we feel that citrate probably also has a role in the biomineralization of the apatite," Schmidt-Rohr said. "It's also been noted in the literature that as an organism ages, the nanocrystal thickness increases and the citrate concentration goes down," Schmidt-Rohr said, "and there's also support from clinical studies that citrate is good for bones," adding that one of the leading supplements for bone strength contains calcium citrate.
"While calcium loss is a major symptom in osteoporosis, the decline of citrate concentration may also contribute to bone brittleness," he said.
Provided by Ames Laboratory