Environmentally friendly rockets
(PhysOrg.com) -- Many rockets, satellites, and spacecraft are driven by hydrazine, sometimes with an oxidizing agent like nitric acid or dinitrogen tetroxide. When filling tanks with these highly toxic substances, technicians must wear full protective clothing—and a failed launch can lead to significant environmental damage. Researchers are thus looking for alternatives that are more environmentally friendly and less toxic, but just as powerful—requirements that are hard to meet in a single material.
Stefan Schneider and his co-workers at the Air Force Laboratory (Edwards Air Force Base, USA) have now introduced a new approach in the journal Angewandte Chemie: special hydrogen-rich ionic liquids that self-ignite in the presence of hydrogen peroxide.
Despite the potential danger, hydrazine is used as a rocket fuel because it delivers high performance, can be stored for a relatively long time, and spontaneously ignites upon contact with an oxidizing agent or a suitable catalyst. The oxidizing agents used as rocket fuels are also dangerous. Dinitrogen tetroxide is less corrosive than nitric acid, but it is toxic and highly volatile. Hydrogen peroxide is a promising alternative because it is less corrosive and leads to much less toxic gas at room temperature. Its decomposition produces only water and oxygen.
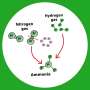
As an alternative to hydrazine as a fuel component, Schneider and his co-workers propose an ionic liquid. Ionic liquids are compounds that consist of ions, namely positive and negatively charged particles, like a salt. However, they are not crystalline; they remain “molten” as a liquid at room temperature. Ionic liquids essentially do not vaporize, which prevents the formation of toxic vapors. It has previously not been possible to produce an ionic liquid that is flammable when partnered with hydrogen peroxide.
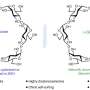
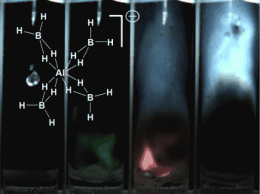
Schneider and his team have now overcome this barrier. The positively charged ion of their ionic liquid is a phosphorus atom bound to four hydrocarbon chains. At the core, however, lies the negatively charged ion made from one aluminum, four boron, and sixteen hydrogen atoms. The hydrogen-rich composition raises the power of the fuel component. “This aluminum borohydride ion can be viewed as a densified form of hydrogen stabilized by metal atoms. In fact, for a given tank size, liquids with this ion contain even more hydrogen than pure liquid hydrogen, without the difficult cooling requirements,” according to Schneider.
In order to test the ignitibility, the researchers applied drops of the novel ionic liquid onto various oxidizing agents. Upon contact with hydrogen peroxide, ignition was nearly instant; with fuming nitric acid it exploded. Says Schneider: “It is thus interesting as a potential component for greener high-performance fuels.”
More information: Stefan Schneider, Green Bipropellants: Hydrogen-Rich Ionic Liquids that Are Hypergolic with Hydrogen Peroxide, Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201101752
Provided by Wiley