May 27, 2011 report
Details of new type of electric car battery released
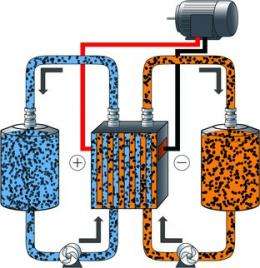
(PhysOrg.com) -- After being spun off from parent company A123 Systems last year; the new offspring, 24M has published a paper in Advanced Energy Materials, ending months of speculation about what it has been working on. It was no secret that the new project was to advance work on a new type of battery that A123 had been working on for a couple of years; namely a battery that could be used to replace the lithium-ion batteries currently used in electric cars. Now, with the paper’s release it's clear that the new battery, similar to a flow battery, uses a liquid material to hold the charge, rather than conventional dry fuel cells, and if successful could do away with a lot of the non-charge holding stuff that makes up nearly three quarters of the bulk of current electric car batteries.
Assisted by a grant from the U.S. Advanced Research Projects Agency-Energy (ARPA-E), to help fund research between the new start-up, MIT and Rutgers University, the new battery, based on research done by Yet-Ming Chiang who is both a professor at MIT and founder of A123 Systems and 24M, if successful, would allow for upsizing of car batteries without adding any non-chargeable material, greatly increasing its density, which would in turn, theoretically greatly reduce the cost of the battery pack in an electric vehicle. Current battery packs now constitute up to a third of the total vehicle price.
The new battery, as described in the paper, uses a sludge-like material contained in storage tanks, rather than dry cells; one positively charged, the other negative. To get the charge from the battery, the materials are pumped through channels allowing ions to move freely between the two and eventually to an external circuit. To facilitate the transfer of electrons from the sludge, nanoscale particles that help to form networks that give the electrons a path to follow were developed and added to the sludge mix. In this type of battery, the amount of storage capacity goes up as the tank size is increased, with no additional materials needed, in sharp contrast to lithium batteries.
The battery is not yet ready for prime time though, as a current model of the battery would be bulky and the electrical conductivity, according to Change, is still far below what would be needed in a real world battery in an actual electrical vehicle; research is still ongoing, as he and his team try to figure out how to increase the concentration of the active materials in the sludge.
More information: Semi-Solid Lithium Rechargeable Flow Battery, Advanced Energy Materials, Article first published online: 20 MAY 2011 DOI: 10.1002/aenm.201100152
Abstract
A new kind of flow battery is fueled by semi-solid suspensions of high-energy-density lithium storage compounds that are electrically ‘wired’ by dilute percolating networks of nanoscale conductor particles. Energy densities are an order of magnitude greater than previous flow batteries; new applications in transportation and grid-scale storage may result.
© 2010 PhysOrg.com




















