Bacterial guests double as bodyguards
The bacterium Escherichia coli can be a scientist’s best friend when it’s being used as a tool for biological research, but some strains of it are better known for their nasty effects on humans as a causative agent in food poisoning.
Infection with the O157:H7 strain of E. coli usually occurs after consumption of meat that has been contaminated by exposure to fecal matter, and the symptoms can range from diarrhea to blood cell loss, encephelopathy and kidney failure. The causative factor is Shiga toxin (Stx), a bacterially secreted compound that makes its way from the intestine into the bloodstream, and ultimately binds to target receptors on cells in the kidney and brain.
According to Hiroshi Ohno of the RIKEN Research Center for Allergy and Immunology in Yokohama, the acquisition of protection against O157:H7 appears to be also related to diet. “In 1996, we experienced an outbreak of O157:H7 in Sakai, Japan,” he says. “Epidemiologic research suggested that children with a history of breast-feeding were more resistant to O157:H7 infection than those with a formula-fed history.”
In addition to nutrition, mother’s milk helps to establish the community of gut bacteria that reside within every human being. These include the bifidobacteria, which have subsequently been associated with conferring O157:H7 resistance. A recent study from a team led by Ohno and Masahira Hattori at The University of Tokyo has revealed the defining characteristics of these defenders of human health.
Knowing who your friends are
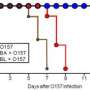
To characterize the protective properties of several different strains of bifidobacteria, the researchers used mice that lacked gut bacteria of their own. Completely ‘germ-free’ mice typically died within a week of exposure to O157:H7, but all animals pre-colonized with a protective strain of Bifidobacterium longum (Fig. 1), termed ‘BL’ survived. Mice carrying BL also showed considerably lower levels of Stx in their bloodstream, and the lining of their intestines appeared healthier. The B. adolescentis strain ‘BA’, however, bought germ-free mice only a few extra days of survival; the intestinal epithelia of these animals exhibited inflammation and evidence of increased levels of cell death following exposure to O157:H7.
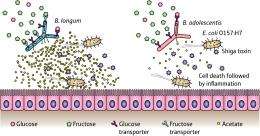
In order to perform a more systematic analysis, Ohno, Hattori and their colleagues examined additional preventive and non-preventive bifidobacteria strains, and determined that these two groups were producing distinct metabolic signatures within the intestine. Gut bacteria in general play a prominent role in assisting in the digestion of carbohydrates, and the researchers learned that the preventive strains tended to generate higher levels of acetate as a byproduct of this process.
Acetate, in turn, switches on the activity of a group of anti-inflammatory genes. Experiments with human colon epithelial cells showed that acetate treatment was protective against the negative effects of Stx, which otherwise induced the formation of ruptures in layers of cultured cells. This suggests that acetate-producing bacteria prevent poisoning by actively preserving the integrity of the intestinal wall and keeping Stx out of the bloodstream.
According to Ohno, these data help connect several important dots in understanding the protective benefits conferred by bifidobacteria in the human gut. “It was well known that acetate is produced by bifidobacteria and can be beneficial for the intestinal epithelium, and it is therefore rather reasonable that acetate is functioning in this role,” he says. “However, I think it would have been difficult to predict from the beginning that acetate is the molecule responsible.”
The secrets of their success
A comparison of the complete genomic sequences of five different bifidobacterial strains by the researchers revealed a subset of genes encoding proteins involved in uptake and metabolism of the sugar fructose that appear to be exclusively present in preventive strains. Accordingly, disabling these genes drastically undermined the protective qualities of such strains. They also determined that it was possible to cut out the bacterial ‘middleman’, and demonstrated that considerable protection against Stx lethality could be achieved by simply feeding mice a diet that was enriched in acetylated starch.
The team points out that all bifidobacteria strains produce acetate during the process of glucose metabolism, protecting the upper stretches of the colon. However, the selectively expressed fructose-metabolizing pathway is likely to provide important protection in the lower region of the colon, a portion of the digestive tract where glucose is likely to be largely depleted (Fig. 2).
Even when the ill effects of O157:H7 infection have been countered, these bacteria will continue to make themselves at home in the gut, although conventional antibiotics should be sufficient to kill them. “I doubt that antibiotics can totally exterminate O157:H7 from the intestine of germ-free mice, as small numbers of O157:H7 remaining in the gut could propagate quickly once antibiotic treatment is terminated,” says Ohno. “[But] humans possess their own ‘commensal’ bacteria that greatly outnumber invading bacteria such as O157:H7, and these would not allow the pathogens to come back after antibiotic treatment.”
Ohno and his colleagues see their bacterial detective work as a powerful proof of concept for understanding the health implications of the close relationship between microbes and their hosts, and for advancing the development of microorganism-mediated ‘probiotic’ therapeutic strategies in the future. “The beneficial effects of probiotics are diverse, and acetate alone could not explain everything; we would therefore like to elucidate the mechanisms underlying other probiotic effects,” he explains, “and we would also like to apply the ‘multi-omics’ approach we took here for analyzing more complex gut ecosystems.”
More information: Fukuda, S., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547 (2011).
Provided by RIKEN