More efficient fuel cell applications via nanotechnology

(PhysOrg.com) -- Engineers at UC San Diego are using nanotechnology to increase the efficiency and enhance the performance of fuel cells, which could boost renewable energy options and reduce toxic emissions.
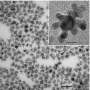
Current fuel cell efficiencies are significantly limited, in part due to an inhibitive reaction from a byproduct. The UC San Diego researchers have synthesized bimetallic nanoparticles (NPs), which are promising materials for fuel cell catalysis due to combined properties from two metals.
Nanoengineering grad student Su-Wen Hsu will highlight this work in his poster titled “Polyelectrolyte-Templated Galvanic Deposition for Bimetallic Nanoparticles” during Research Expoon April 14.
Hsu and his research team are using bimetallic NPs to optimize the performance of current fuel cell catalysts by enhancing the catalyst activity and selectivity.
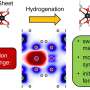
A catalyst is a substance that increases the rate of a chemical reaction without being consumed or chemically altered, and does this by reducing the energy needed for the reaction to proceed. In order for fuel cells to become a viable economical solution, their catalytic processes must be optimized. For example, splitting water into hydrogen and oxygen to feed a fuel cell is a highly desirable process, but catalytic activity for this system needs to be improved.
“We modified the surface charges of Ag NPs using differently charged polyelectrolytes and used these as templates for galvanic displacement with Au,” Hsu said. “Positively charged NPs generated hollow bimetallic shell structures, and negatively charged NPs generated porous and aggregated bimetallic structures.”
“The synergistic effect of Ag/Au NPs makes them excellent catalysts for CO oxidation and may lead to potential application in fuel cells,” added Hsu, whose advisor is UC San Diego nanoengineering professor Andrea Tao. “The ability to tailor NP morphology and composition will allow us to evaluate these bimetallic NPs as potential nanocatalysts for low-temperature reaction."
For Hsu and his team, they are one step closer to advancing the development of fuel cells, which may be used to power production in portable, stationary and transportation applications like consumer electronics, residential units and specialty vehicles. Nanotechnology is expected to improve material properties, the functionality and performance of components, and decrease the price of fuel cells.
“There are many special properties in nano-size material compared with bulk material. This is the most interesting part in nanoengineering,” Hsu said. “I hope I can understand this area more. In the future, we will measure some properties of the bimetallic nanoparticles to prove those bimetallic NPs can be used in catalyst in different areas."
Provided by Jacobs School of Engineering