Small scale chemistry could lead to big improvements for biodegradable polymers
.jpg)
(PhysOrg.com) -- Using a small block of aluminum with a tiny groove carved in it, a team of researchers from the National Institute of Standards and Technology and the Polytechnic Institute of New York University is developing an improved “green chemistry” method for making biodegradable polymers. Their recently published work is a prime example of the value of microfluidics, a technology more commonly associated with inkjet printers and medical diagnostics, to process modeling and development for industrial chemistry.
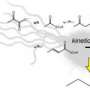
“We basically developed a microreactor that lets us monitor continuous polymerization using enzymes,” explains NIST materials scientist Kathryn Beers. “These enzymes are an alternate green technology for making these types of polymers—we looked at a polyester—but the processes aren’t really industrially competitive yet,” she says. Data from the microreactor, a sort of zig-zag channel about a millimeter deep crammed with hundreds of tiny beads, shows how the process could be made much more efficient. The team believes it to be the first example of the observation of polymerization with a solid-supported enzyme in a microreactor.
The group studied the synthesis of PCL (Polycaprolactone), a biodegradable polyester used in applications ranging from medical devices to disposable tableware. PCL, Beers explains, most commonly is synthesized using an organic tin-based catalyst to stitch the base chemical rings together into the long polymer chains. The catalyst is highly toxic, however, and has to be disposed of.
Modern biochemistry has found a more environmentally friendly substitute in an enzyme produced by the yeast strain Candida antartica, Beers says, but standard batch processes—in which the raw material is dumped into a vat, along with tiny beads that carry the enzyme, and stirred—is too inefficient to be commercially competitive. It also has problems with enzyme residue contaminating and degrading the product.
By contrast, Beers explains, the microreactor is a continuous flow process. The feedstock chemical flows through the narrow channel, around the enzyme-coated beads, and, polymerized, out the other end. The arrangement allows precise control of temperature and reaction time, so that detailed data on the chemical kinetics of the process can be recorded to develop an accurate model to scale the process.
“The small-scale flow reactor allows us to monitor polymerization and look at the performance recyclability and recovery of these enzymes,” Beers says. “With this process engineering approach, we’ve shown that continuous flow really benefits these reactors. Not only does it dramatically accelerate the rate of reaction, but it improves your ability to recover the enzyme and reduce contamination of the product.” A forthcoming follow-up paper, she says, will present a full kinetic model of the reaction that could serve as the basis for designing an industrial scale process.
While this study focused on a specific type of enzyme-assisted polymer reactions, the authors observe, “it is evident that similar microreactor-based platforms can readily be extended to other systems; for example, high-throughput screening of new enzymes and to processes where continuous flow mode is preferred.”
More information: S. Kundu, et al. Continuous flow enzyme-catalyzed polymerization in a microreactor. J. Am. Chem. Soc. DOI:10.1021/ja111346c
Provided by National Institute of Standards and Technology