Modeling retinitis pigmentosa with iPS cells
Retinitis pigmentosa (RP) is a cluster of genetically determined eye disorders that cause visual defects such as night blindness and narrowing of the field of vision, due to progressive loss of rod photoreceptors. As many as 45 different genes have been linked to the inheritance of this disease, which suggests diversity in etiology and makes development of a standardized animal model problematic. Thus, despite a range of clinical trials of nutritional and drug-based interventions for RP, the disease remains untreatable. Better platforms for modeling the disease and testing drug candidates in vitro are urgently needed.
New work by Zi-Bing Jin and colleagues in the Laboratory for Retinal Regeneration (Masayo Takahashi, Team Leader) looks to add a set of powerful new tools for those searching for treatments for RP. In an article published in PLoS One, the team reports the generation of induced pluripotent stem cells (iPSCs) from patients carrying mutations in several RP-associated genes, and the subsequent differentiation and characterization of rod photoreceptors from these genetically distinct, patient-derived pluripotent cells.
After obtaining informed consent from five RP patients with distinct mutations in the RP1, RP9, PRPH2, or RHO gene, the team took samples of skin cells and used the fibroblasts as a starting point for generating iPSCs. Using the classic reprogramming cocktail of Oct4, Sox2, Klf4, and c-Myc delivered via a retroviral vector, Jin and colleagues generated cell lines from each patient and verified their conversion by tests for appropriate morphology, genetic and karyotypic integrity, and teratoma formation.
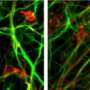
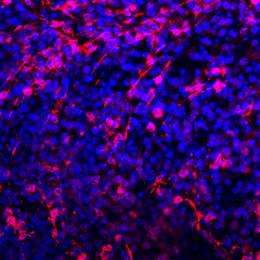
Using these iPSCs, the team next generated photoreceptors carrying the genetic signatures of each of the five patient donors using a previously established stepwise protocol that steered the cells over four months in culture from an undifferentiated ES cell-lie state through retinal progenitor, and photoreceptor precursor stages to the desired rod photoreceptor phenotype. The differentiated cells were shown to express the rod photoreceptor marker rhodopsin at high levels, and to have similar electrophysiological function.
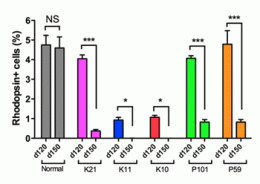
Interestingly, rod photoreceptor cells generated from iPSC colonies carrying RP-linked mutations showed a tendency to degenerate, while cone photoreceptors and bipolar cells derived from the same iPSCs were stable. The mechanisms underlying this instability turned out to be dependent on the affected gene. Rod photoreceptors generated from iPSCs with a mutation in the RP9 gene showed evidence of DNA oxidation, while those from iPSCs with a mutation in the rhodopsin gene showed signs of stress on the endoplasmic reticulum, the site of protein synthesis.
As an initial proof-of-concept test of their suite of RP-specific rod photoreceptors in drug validation, Jin and colleagues examined the effects of antioxidant vitamins in preventing degeneration of these cells in vitro. Ascorbic acid, α-tocopherol, and β–carotene have all been tested in clinical trials as anti-oxidant therapies for RP, but all had not proved very effective. When the team tested these on individual cells lines by treating them with one of the three antioxidants for seven days at around the stage at which rod photoreceptor degeneration occurs, they found that α-tocopherol increased cell survival in the lines generated from two patients both carrying mutations in RP9. The same treatment was ineffective in cells from other patients, and ascorbic acid and β–carotene had no effect in any of the lines. These results, which show the efficacy of α-tocopherol in promoting survival in RP9 rod photoreceptors, highlights the potential of patient-derived induced pluripotent stem cells in the study of disease mechanisms and in vitro testing of treatment approaches.
“Using iPSCs from cells donated by RP patients with different underlying genetic mutations, we were able to show that rod photoreceptors generated from these cells underwent apoptosis in vitro, and showed differing responses in a genetically determined manner to drug treatment,” says Takahashi. “This is one of the first reports to demonstrate that patient-derived iPSCs may be useful in personalized medicine, as differential responses within a genetically diverse study group will tend to be lost in the crowd. Future improvements in differentiation protocols, screening techniques, cost and efficiency and the establishment of methods for isolating photoreceptors may open up new possibilities for the use of these cells in drug screening.”
More information: Jin ZB, et al. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS One. 2011 Feb 10;6(2):e17084. www.ncbi.nlm.nih.gov/pubmed/21347327?dopt=Citation
Provided by RIKEN