Sorted building blocks: Poly(propylene carbonate) stereogradient
(PhysOrg.com) -- The properties of polymers—long chain molecules from which plastics are made—depend on the type of individual building blocks in them, as well as the order they are in and how they are arranged in space. Although the order of the components can easily be controlled, control of their spatial arrangement, called stereochemistry, remains one of the biggest challenges in polymer chemistry. Kyoko Nozaki and a team from the University of Tokyo report in the journal Angewandte Chemie that they have made the first poly(propylene carbonate) with polymer chains built up in the form of a gradient of two stereochemically different propylene building blocks.
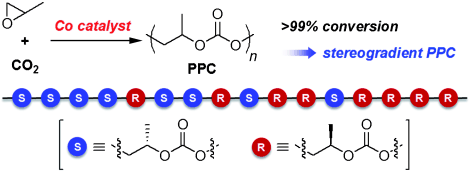
Poly(propylene carbonate) is used as a binding agent and as a component of biodegradable plastics. It is made from propylene oxide and carbon dioxide in a catalytic process. Propylene oxide contains three carbon atoms, two of which form a ring together with an oxygen atom. This ring opens during polymerization. Propylene oxide exists in two forms that are mirror images of each other; these are designated as the S and R stereoisomers.
Poly(propylene carbonate)s that are made primarily of one of the two forms or have both forms in an alternating pattern have been made before. Nozaki’s group has now been the first to synthesize both a stereoblock and a stereogradient. A stereoblock copolymer is a chain, half of which is made of the S form and the other half of the R form. In a stereogradient copolymer, the composition changes gradually from the S form to the R form.
Making a block copolymer is theoretically relatively easy because use of an asymmetric catalyst causes one of the two forms of building block to be used preferentially, so it is built into the polymer chains first; the less favorable form is incorporated afterward. In the case of poly(propylene carbonate), however, this process isn’t so trivial because once the favored form of the propylene oxide is converted to a polymer, the other form decomposes instead of polymerizing. The Japanese scientists found a special asymmetrical cobalt complex that allows nearly complete conversion to the polymer. Although the catalyst prefers the S form, it also ensures that it is more favorable for the R form to polymerize than to decompose.
The researchers experimented further with variations on the cobalt complex. A special ammonium side branch on a ligand brought success: It balances the degree of preference of the catalyst for the S form over the R form so that the R form begins to be incorporated into the polymer chain as the amount of the S form decreases. This allows the formation of the stereogradient copolymer. Interestingly, both of the new types of poly(propylene carbonate), stereoblock and stereogradient, are significantly more heat-tolerant than pure S or R polymers or mixtures of the two.
More information: Kyoko Nozaki, Synthesis of Stereogradient Poly(propylene carbonate) by Stereo- and Enantioselective Copolymerization of Propylene Oxide with Carbon Dioxide, Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201007958
Provided by Wiley