Proteins find their way with address label and guide
Most newly produced proteins in a cell need to be transported to the proper place before they can be put to work. For proteins to find their way, they have a built-in signal linked to them, a kind of address label. Moreover, they are helped by a particle that guides them to the cell membrane. In a new study published in the journal Nature Structural and Molecular Biology, researchers at Umea University in Sweden show how this interaction works.
Calculations indicate that each human cell contains roughly a billion protein molecules. In other words, it's crowded inside the cell, and order must be maintained. What's more, newly generated proteins often need to be transported from the place they were produced to the place they are to perform their tasks. These proteins have a kind of address label, a signal sequence, that specifies what place inside or outside the cell they need to be transported to. This transport must function flawlessly if order is to be maintained in the cell, but also for the cell to be able to communicate with its surroundings. If a protein winds up in the wrong place, it can lead to serious disorders like cystic fibrosis.
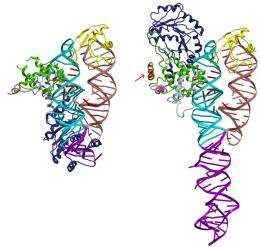
The capacity to transport proteins in most cases is directly linked to the function of the SRP, the signal-recognizing particle. The SRP binds to the signal sequence and guides it and the attached protein to the cell membrane. A key question for these researchers has been how the interaction between the signal sequence and SRP works in detail.
The Umeå scientists have managed to create a detailed picture of the first step in this protein transport by studying a complex of a signal sequence that is bound to the SRP. The technology they used is called x-ray crystallography. The group has shown the basic structure of the SRP in several previous studies SRP. Thanks to these studies, they were now able to directly compare the SRP structure with and without the guiding signal sequence.
”The structural changes were considerably greater than what was previously predicted. They provide us with detailed explanations of what role SRPs play in protein transport. These structural specifications can also serve as a model of how SRPs function at various levels during protein transport," explains Elisabeth Sauer-Eriksson, professor at the Department of Chemistry.
Now these researchers are moving on to try to investigate the next transport mechanism. For instance, they want to answer questions about what prompts the bound signal sequence to let go of the SRP and how the signal sequence, and the protein it is attached to, can make its way through the membrane.
More information: "Structural basis of signal-sequence recognition by the signal recognition particle," Tobias Hainzl, et al. Nature Structural and Molecular Biology.
Provided by Umea University

















