Microbially produced ferrous iron may decrease technetium concentrations in groundwater
(PhysOrg.com) -- The long-lasting radionuclide technetium is transported through the subsurface near former nuclear production and processing sites, moving toward rivers and lakes. But its journey can come to an abrupt end if it hits an area containing high levels of reduced iron generated by microbes.
Scientists from Pacific Northwest National Laboratory recently found that microbially generated iron creates significant roadblocks for the pollutant. They determined that in the presence of commonly occurring oxidized iron minerals, indirect technetium reduction by microbially generated ferrous iron, or Fe(II), may be favored over direct technetium reduction by bacteria, making the technetium as much as 10 times less soluble.
Technetium-99 (99Tc), a radioactive by-product of nuclear production and processing, has a half-life of 200,000 years. Its common oxidized form, Tc(VII)O4-, or pertechnetate, is highly mobile in subsurface sediments and groundwater. This makes it of concern at Department of Energy plutonium production sites such as the Hanford Site in Washington State, and others in Paducah, KY, and Portsmouth, OH.
Fortunately, pertechnetate can be chemically reduced to less mobile forms by subsurface minerals containing reduced or ferrous (Fe2+) iron. The ferrous iron content of the subsurface can, in turn, be increased by metal-reducing bacteria, such as Shewanella, Geobacter, and Anaeromyxobacter.
The tenfold difference in technetium solubility is important, because the Environmental Protection Agency limit for 99Tc in drinking water is extremely low—near the solubility value for the chemically reduced form of Tc, TcO2 (technetium dioxide). Furthermore, the PNNL researchers found that direct biological reduction of technetium by metal-reducing bacteria generated small-particle technetium colloids that could be highly mobile. Previous research revealed that technetium oxide associated with minerals can be resistant to reoxidation and mobility.
Pertechnetate can be reduced to technetium oxide or to various Tc(IV) chemical phases by microbial enzymes that can generate low redox potential, such as hydrogenase or c-type cytochromes. Various forms of reduced inorganic ions, such as ferrous iron or sulfide are also produced and these, have the potential to reduce pertechnetate. The effectiveness of these reductants is extremely dependent on their chemical speciation and mineral form.
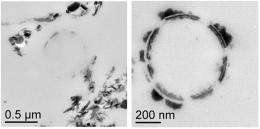
Using several dissimilatory metal-reducing bacteria, the PNNL team examined the bioreduction of pertechnetate in the presence and absence of the poorly crystalline iron oxide. They examined the resulting bioreduced materials by transmission electron microscopy, X-ray absorption spectroscopy, microcapillary X-ray diffraction, and traditional wet-chemical analytical methods. Technetium solubility was determined by sequential ultrafiltration, solvent extraction, and liquid scintillation counting.
The researchers are now examining the enzymatic reduction of technetium by hydrogenase and cytochromes to gain insight into the properties of biogenic technetium oxide and the electron transfer mechanisms responsible for the reduction. Simultaneously, they are examining the biogeochemical transformation reactions in representative Hanford Site sediments and the microbial and geochemical catalysts responsible for technetium reduction.
More information: Plymale AE, JK Fredrickson, JM Zachara, AC Dohnalkova, SM Heald, DA Moore, DW Kennedy, MJ Marshall, C Wang, CT Resch, and P Nachimuthu. 2011. "Competitive Reduction of Pertechnetate (99TcO4-) by Dissimilatory Metal Reducing Bacteria and Biogenic Fe(II)." Environmental Science & Technology 45(3):951-957. DOI:10.1021/es1027647
Provided by Pacific Northwest National Laboratory
















