High-pressure division
To be able to divide, a cell must first of all create enough space for itself in the tissue. How cells do that was a mystery, until now. Researchers from the Department of Biosystems Science and Engineering at ETH Zurich have now discovered that cells "inflate" themselves generating hydrostatic pressure during division. By doing this cells develop an enormous force, which they use to push other cells aside.
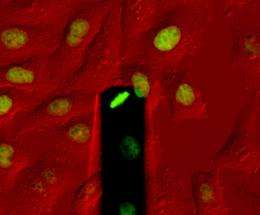
Tissues, e.g. skin or muscles, consist of a dense network of cells in close contact with one another. If an individual cell wants to divide into two daughter cells, it must first detach itself from its neighbouring cells and round up into a sphere. At the same time it increases its volume, for which it needs additional space. How the cell can achieve this dramatic change amongst the densely congregated cells was unknown until now.
Researchers led by Daniel Müller, Professor of Biophysics at ETH Zurich, have now solved this problem, in collaboration with researchers from Dresden. Their results, which they have published in the scientific journal Nature, show that animal cells absorb water during division. As a result they build up hydrostatic pressure, which inflates them like a balloon. This generates a force that can push aside the surrounding tissue. Daniel Müller is convinced that “This means cells have developed an active process to create space for themselves.”
Miniature muscleman
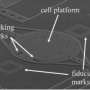
To measure the force cells develop during division, Martin Stewart a research team member used a scanning force microscope on which there is a silicon spring only a few hundredths of a millimetre long. The researchers positioned the spring above a cell that had grown on the bottom of a Petri dish and had just started to divide. At first the spring did not touch the cell, but as division progressed the cell expanded more and more until it pressed the spring upwards. The force that is measurable in this process is only about 0.0000001 Newton. Although that sounds small, it is very large on the scale of a single cell. It corresponds approximately to the force a person would need to hold back an elephant.
In further experiments the researchers tried to find out how the cell succeeds in generating so much force. They did this by searching for proteins that become particularly active during cell division. Eventually they discovered a transport protein in the cell envelope that transports sodium ions into the cell interior, thus regulating the salt balance. They observed that a particularly large amount of sodium is transported when a cell divides. This generates an osmotic effect that causes water to flow into the cell’s interior. The more water flows in, the more the cell expands.
Soft shell, hard core
However, that on its own would not be enough to enable the cell to exert a force on its surroundings, because its envelope would be too soft and unstable. Daniel Müller compares it to an inflated bicycle inner tube that gives way when pressed. The tube only becomes taut due to the tyre, which keeps it in shape. In cells this function is carried out by the cell skeleton, consisting of a network of actin molecules which are also proteins. The skeleton is located immediately beneath the cell envelope and offers it stability.
When the researchers used a chemical substance to destroy the actin network of a dividing cell, the cell expanded even further. Conversely, it shrank when the actin was left intact but the inflow of water was blocked. From this the scientists concluded that two forces interact in opposite directions: one directed outwards, which inflates the cell, and an opposing force directed inwards, which causes the pressure to increase.
So far Daniel Müller’s team has only studied individual growing cells, but not those inside tissue. “Our measurement method is not yet suitable for that”, he explains. However, studies by microscopy have yielded evidence that the same processes also take place in tissues. Moreover, division only succeeds if sufficient space is available. The researchers infer this from an experiment in which they used the silicon spring to exert so much pressure on the cell that it was unable to expand. More than seventy percent of the cells treated in this way did not complete the division and died.
However, sooner or later the cell must also release the pressure that it has built up during the division process. The researchers still cannot say how it knows when to do this. Müller suspects that “There must be a signal for it”. He and his team are already tracking this down in a new project.
More information: Martin P. Stewart, et al: Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature Advanced online publication (2 January 2011), doi:10.1038/nature09642
Provided by ETH Zurich