A greener path for the production of a vital chemical
(PhysOrg.com) -- Nanoparticles of gold and palladium (Au-Pd) could lead to a more efficient and environmentally friendly way of producing benzyl benzoate, a chemical compound used widely in the food, pharmaceutical and chemical industries whose applications include a fixative for fragrances, a food additive and a solvent for chemical reactions.
The most common method of producing benzyl benzoate is to react benzoic acid with benzyl alcohol. It can also be generated from benzaldehyde. All three starting materials are derived from toluene, a component of crude oil. The manufacture of benzyl alcohol and benzaldehyde requires the use of halogens and acidic solvents, whereas benzoic acid is produced via a more environmentally friendly liquid phase cobalt-catalyzed reaction.
A research team led by Graham Hutchings, professor of chemistry at Cardiff University in Wales in the United Kingdom, and Christopher Kiely, professor of materials science and engineering at Lehigh, has found a way of producing benzyl benzoate directly from toluene in a solvent-free, single-step process using Au-Pd nanoparticles to catalyze the reaction.
“By optimizing the Au-Pd ratio in the nanoparticle, as well as the reaction conditions, we were able to achieve conversion rates of over 95 percent with no conversion to carbon dioxide,” says Hutchings.
Shining a light on particle size and catalytic activity
The researchers reported their finding Jan. 14 in Science magazine in an article titled “Solvent-Free Oxidation of Primary Carbon-Hydrogen Bonds in Toluene Using Au-Pd Alloy Nanoparticles.” The article was coauthored by Hutchings and Kiely and 10 other researchers, including Ramchandra Tiruvalam, a Lehigh Ph.D. candidate working with Kiely.
Rather than making the catalysts by conventional support impregnation techniques, the researchers chose a preparation route that involved the sol-immobilization of Au-Pd colloids using amorphous carbon and titanium oxide supports. This technique offers much greater control over particle size and composition than do conventional methods.
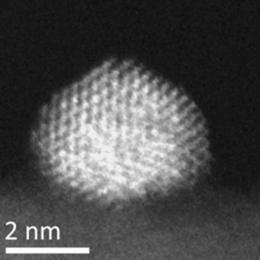
Transmission electron microscopy (TEM) studies carried out by Tiruvalam revealed that the average particle sizes were very similar, 3.3 nanometers on carbon and 3.5nm on titanium oxide.
“Despite having a very similar particle-size distribution, the Au-Pd/carbon samples were found to have approximately double the catalytic activity of the Au-Pd/titanium oxide samples,” says Kiely, who directs the Nanocharacterization Laboratory in Lehigh’s Center for Advanced Materials and Nanotechnology.
“This suggests that simple metal surface area considerations are not dominating the catalytic activity.”
Achieving stability and reusability
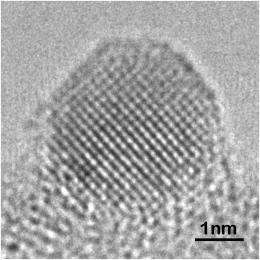
Using Lehigh’s aberration-corrected TEM, Tiruvalam was able to show that the particles were indeed Au-Pd alloy particles, that those on the titanium oxide were highly faceted and tended to form a flat interface with the support, and that those on the carbon were much more rounded.
“The difference in catalytic activity may be related to differences in the number of low coordination number edge and corner sites available,” explains Kiely. “The more rounded ‘rougher’ particles on the carbon support have significantly more of these sites than the flatter particles on the titanium oxide support.”
In a final set of experiments, the researchers were able to demonstrate that the Au-Pd/carbon catalysts showed no loss of activity after use and that there was little change in particle shape and size after extended reaction periods.
“It is clear that these highly active catalysts are both stable and reusable,” says Kiely.
More information: www.sciencemag.org/content/331/6014/195.full?rss=1
Provided by Lehigh University