Expert discusses using small-pore zeolites to remove troublesome pollutant from exhaust
(PhysOrg.com) -- While the reactions that eliminate smog-causing nitrogen oxides from automotive tail pipes appear straightforward, there is far more happening than the standard freshmen textbook shows. Professor Raul Lobo of the University of Delaware recently shared his progress in understanding a new generation of catalysts to eliminate nitrogen oxides. His talk was part of the Frontiers in Catalysis Science and Engineering Seminar Series. The seminars, held at Pacific Northwest National Laboratory, allow experts to share novel ideas and studies.
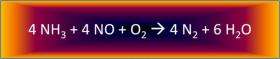
For years, scientists have studied zeolites to drive reactions that convert nitrogen-oxygen compounds, also known more simply as NOx, to pure nitrogen gas and water. Zeolites are silica-alumina-based materials where chemicals mix and reactions take place inside tiny pores. Using ion exchange methods, active catalyst metals such as copper can be added to the zeolites, creating the active sites for the catalytic chemistry.
While this porous catalyst material is efficient in lab settings, it often fails at higher temperatures and in the presence of hydrocarbon and/or water vapors. These flaws make applying zeolite catalysts to truck and automotive systems a considerable challenge.
At the University of Delaware, Lobo is leading research into zeolite catalysts that can overcome these flaws. For example, Lobo's team has provided important new information about the structure of a new small-pored zeolite containing copper. This catalyst is known as Cu-SSZ-13.
After initial studies of the catalyst's structure, the team began seeing how the material responded to conditions typical of an exhaust system. In a series of experiments, the team showed that Cu-SSZ-13 is thermally stable performing at temperatures up to 500°C. Other materials structurally related to Cu-SSZ-13 also appear to be both active and stable catalysts for this reaction.
The copper-substituted material also stands up well to water, always present in automobile exhaust. In comparing microscopy images of Cu-SSZ-13 to another commonly used copper-containing zeolite, Cu-ZSM-5, the team saw that the ZSM-5 version becomes pitted and pruny when exposed to water. The Cu-SSZ-13 does not change appreciably.
"Raul Lobo's catalysis research is opening new doors into our understanding of zeolites," said Dr. Charles H.F. Peden, Interim Director of PNNL's Institute for Interfacial Catalysis." With the IIC's mutual interests in these materials, I'm especially delighted that he came here to see our research and speak with the Institute's scientists."
Provided by Pacific Northwest National Laboratory
















