Redox response properties of largest-ever polymeric o-phenylenes uncovered
New findings by researchers at RIKEN and the Japan Science and Technology Agency (JST) have shed light on the remarkable electrochemical response properties of an elusive class of molecular helix structures, charting a new path in the design of molecular machines and devices.
Among the most ubiquitous structural motifs in nature, helices play an essential role in a wide range of biological processes. The capacity of certain helix structures to respond to external stimuli by changing shape, in particular, offers key insights in the design of functional molecular devices. As of yet, however, few such structures have been identified that respond to electrochemical inputs, one of the most important types of stimuli.
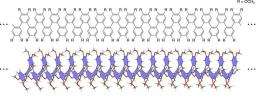
Now a class of helical structures has been found to do this, and more. o-Phenylenes are densely-packed chains of phenylene (C6H4) compounds linked together at their ortho positions by heavily-angled connections. Despite potentially rich conformational behavior, o-phenylenes are difficult to study and have been all but forgotten since their discovery more than 50 years ago.
In a paper in Nature Chemistry, the RIKEN/JST research group demonstrates a method for synthesizing polymeric o-phenylenes on scales never before observed, the largest reaching some 48 phenylene units. Problems of electrochemical instability which plagued earlier studies are solved by introducing a nitrogen group to the end of the o-phenylene chain, enabling first-ever exploration of o-phenylene oxidation-reduction response.
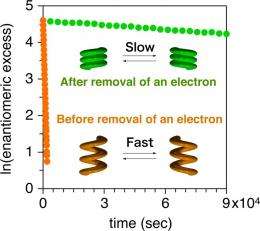
Experiments with the new o-phenylenes revealed intriguing results. In solution, the helices depart from their folded form to undergo rapid inversion between clockwise and anti-clockwise orientations, yet when they crystallize, they converge uniformly to only one orientation, in a rare process called chiral symmetry-breaking. Removal of a single electron, meanwhile, converts helices across the entire solution to a more compact form, slowing the inversion rate by a factor of more than 450.
Through its parallel to permanent and long-lasting memory, this unique form of conformational rigidity alterable by electrical inputs offers a completely new design concept for nanotechnology, opening new avenues for the design of molecular wires and other nano-scale devices.
More information: Eisuke Ohta, et al. Redox-responsive molecular helices with highly condensed p –clouds. Nature Chemistry (2010). DOI:10.1038/NCHEM.900
Provided by RIKEN


















