Key Parkinson's clue could be protein aggregate
(PhysOrg.com) -- Proteins perform almost every function our bodies require for life. But, they also can misbehave in myriad ways. By retracing the history of each abnormal reaction, biochemists aim to determine the events that lead to disease and to intervene in the process.
Collaborative research between Ithaca-based Cornell applied physicists and biochemists at Weill Cornell Medical College has yielded new clues into what happens when the Parkinson's disease-associated protein alpha-synuclein undergoes abnormal aggregation. These findings are published in the Nov. 2 edition of Proceedings of the National Academy of Sciences (online Oct. 14).
Parkinson's disease patients have dense lesions in their midbrains called Lewy bodies, which involve aggregates of alpha-synuclein. But it is still unclear whether Lewy bodies are a symptom of the disease or are themselves responsible for cell death. Many researchers surmise that smaller clusters (known as oligomers or aggregates) of alpha-synuclein protein could be responsible for initiating neurodegeneration.
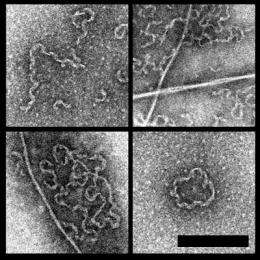
The Cornell group, led by applied and engineering physics professor Watt W. Webb and Weill Cornell biochemistry professor David Eliezer, aimed to shed light on structural changes in alpha-synuclein. Using chemical solutions of fluorinated alcohol to trigger protein structural transitions, the researchers observed the formation of irregular, helical aggregates that may be similar to formations in the brain of Parkinson's disease patients. These structures, some long and thin, and others inter-wound or spooled, could suggest alternative pathways to alpha-synuclein aggregation in the brain.
"These ringlike annular aggregates have been seen with atomic force microscopy before, and people have been interested in them for a long time," said Valerie Anderson, first author and a graduate student in Webb's lab. The research shows that the aggregates maybe involved in infiltrating healthy cells and causing disease, which could result in toxicity. (See accompanying electron nanoscopic images.)
The new Cornell experiments paint stunning visual evidence of a wide array of protein aggregates with varying molecular structures, some of which might be key to understanding Parkinson's disease. In addition, the researchers identified early events in the assembly of these structures. By examining the interaction of types of polarized light with the alpha-synuclein protein, they observed rearrangements of the protein on the molecular level prior to aggregation. Additional changes in the molecular conformation occurred when the proteins stuck together; alpha-synuclein adopts a helical structure that converts to an aligned, sheetlike molecular assembly early in the aggregation process.
Understanding disease is like solving a mystery -- a million clues yield nothing, but the right one could lead to new treatments. These latest results shed light on early events that occur when alpha-synuclein behaves badly, although more research must be done to determine whether similar events take place in the brains of Parkinson's disease patients.
The researchers made use of microscopy and spectroscopy equipment at the Cornell Center for Materials Research, which is supported by the National Science Foundation. The research was also funded by the National Institutes of Health and the NSF Science and Technology Center program.
Provided by Cornell University















