Chemists design molecule that responds to stimuli
(PhysOrg.com) -- The venus flytrap plant captures its prey when it senses the presence of an insect on the tips of its leaves. An amphiphilic molecule designed by chemists at The City College of New York acts in a similar manner by changing its structure when heated slightly and, then, reverting to its original form when cooled.
The finding, reported in the journal Angewandte Chemie, points toward the possibility of designing adaptive soft materials in the lab that take their cues from how nature responds to stimuli, said Dr. George John, associate professor and corresponding author.
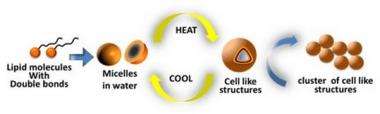
Professor John and colleagues designed the molecule, which has both water-adhering and water-repelling ends, from cardanol, a naturally available material found in cashew nut shell liquid. When mixed with water, the molecules formed a self-assembled structure called a micelle with a water-adhering exterior and water-repelling interior.
Warming the micelles to 50 degrees Celsius caused them to take on a three-dimensional structure known as a vesicle that was larger – 200 – 300 nm in diameter – and viscous, much like oil. "The molecules would stick together, similar to caviar," Professor John said. "When we touched the material with a glass rod, we could draw it out in a thin strand, much like glue."
Allowing the material to cool resulted in the molecules reverting to their original micellar structure. When they were reheated, they would again take on the viscous form.
The change in structure resulted because, while heating caused the micelles to rearrange, they began to interlock in a bi-layer arrangement and eventually undergo curvature. Directional hydrogen bonding of the amide linkages and stacking of the aromatic ring groups, further stabilized the assembly.
The objective of the research is to study responsive systems, Professor John said. "If we can understand the influence of saturation at the bi-layer stage, we can regulate the adaptive response to stimuli." This will require investigating the number of micelles needed in a mixture and where they need to be positioned.
Provided by City College of New York
















