Saving platinum: Monolayer of platinum on tungsten carbide catalyzes electrolytic production of hydrogen
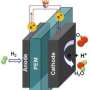
(PhysOrg.com) -- Hydrogen is one of the most promising fuels of the future. Whether powered by wind or sun energy, electrolysis of water is the method of choice for producing hydrogen without emission of carbon dioxide. The character and properties of the hydrogen-producing catalyst, usually platinum, are of critical importance for the efficiency and cost of the electrocatalytic system.
In the journal Angewandte Chemie, Jingguang G. Chen and a team at the University of Delaware (USA) have now introduced a new method for saving on platinum without losing efficiency: they deposit a single layer of platinum atoms onto an inexpensive tungsten carbide support.
Splitting water by electrolysis to produce hydrogen only works efficiently if the cathode, the cell’s negative electrode, is equipped with an efficient catalyst. Platinum is the material of choice because of its high activity -- unfortunately it is very expensive, currently costing around 52 dollars a gram. “Its high price and limited availability are the biggest stumbling blocks on the way to the mass production of hydrogen through electrolysis,” explains Chen.
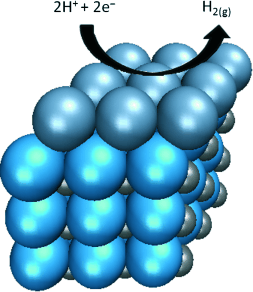
Current attempts to save on platinum by depositing platinum particles onto a support, have not been efficient enough. The platinum atoms often settle too far inside the porous support and are shielded from the reaction. Says Chen, “Our aim was to deposit a single layer of platinum atoms onto an inexpensive planar support so that all the platinum atoms can participate in the reaction.”
The problem with this method is that if such a monolayer of metal atoms is deposited onto a support, the atoms interact with the substrate. The electronic structure of the atoms can change because the distances between the individual atoms in the layer can be different from those in the pure metal. In addition, bonding between the platinum and atoms of the support can lead to undesired effects. This can greatly disrupt the catalytic properties.
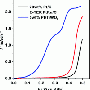
Chen and his team selected tungsten carbide as a carrier. This inexpensive material has properties very similar to those of platinum. They deposited thin films of tungsten carbide onto a tungsten substrate and added platinum atoms by vapor deposition. The chemical and electronic properties of these atomic platinum monolayers on tungsten carbide did not differ significantly from those of a block of pure platinum. The catalytic efficiency of the supported platinum monolayer is also correspondingly strong.
“Tungsten carbide is the ideal substrate for platinum,” says Chen. “It is possible to use significantly smaller amounts of platinum, which reduces the cost—possibly not just for water electrolysis, but also in other platinum-catalyzed processes.”
More information: Jingguang G. Chen, Low-Cost Hydrogen-Evolution Catalysts Based on Monolayer Platinum on Tungsten Monocarbide Substrates, Angewandte Chemie International Edition, dx.doi.org/10.1002/anie.201004718
Provided by Wiley