Unique Uranium Source in Naturally Bioreduced Sediment
(PhysOrg.com) -- A recently published Pacific Northwest National Laboratory study of a naturally bioreduced sediment sample from a former uranium mill tailings site reveals insights that enhance understanding of the long-term persistence of uranium in groundwater. The study provides the first-ever evidence of a useful pyrite mineral formation within the sample.
This mineral form is called "framboidal" because its surface resembles a framboise (French for raspberry). The study presents data showing that framboidal pyrite has an affinity for uranium, decreasing its transport via groundwater. The sample originated from a U.S. Department of Energy Integrated Field Research Challenge site at Rifle, Colorado.
This new understanding will help in developing strategies for keeping the contaminant out of aquifers and away from humans, animals and crops.
"We are trying to determine what kind of minerals in soil have a higher affinity for uranium; that is, what it will stick to," said Dr. Nikolla Qafoku, a PNNL geochemist, who with Dr. Ravi Kukkadapu, an EMSL chemist, is lead author of the paper, which appears in Environmental Science & Technology. EMSL is the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by DOE and located at PNNL.
Past research on pyrite crystals was done on synthesized pyrite made in the lab. These studies demonstrated that uranium adsorption and partial reduction occur on the surfaces on both unweathered and weathered (oxidized) pyrites. However, no such studies have been done with naturally occurring pyrite. High concentrations of uranium in pyrite, including framboidal pyrite in naturally bioreduced alluvial—or silty—sediments, have not been previously reported.
Naturally bioreduced sediment makes up 10% of an alluvial aquifer adjacent to the Colorado River, within the Rifle site. Past and ongoing research has demonstrated that bioreduced sediment has higher amounts of uranium, Fe(II), organic carbon and sulfides.
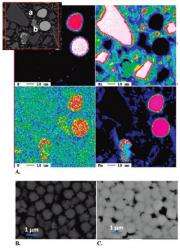
The researchers used a series of wet chemical extraction methods and microscopic and spectroscopic techniques on the Rifle sediment sample to determine its mineralogy and the affinity of uranium for different minerals. At EMSL, they used X-ray diffraction, scanning electron microscopy, transmission electron microscopy and Mössbauer spectroscopy to characterize the iron in the sediment. The results initially showed that the iron sulfide in the form of framboidal pyrite is abundant in the fine-grained fraction of this sediment. Electron microprobe analysis produced elemental mappings showing that uranium was associated with framboidal pyrite.
"The results were really exciting," said Qafoku, "because this was the first time that uranium was found to be associated with naturally occurring framboidal pyrites in alluvial sediments." X-ray spectroscopic analyses conducted at the Advanced Photon Source at Argonne National Laboratory confirmed that framboidal pyrite uranium occurred in both oxidized and reduced forms. This is important because the mobility of contaminant uranium depends strongly on its valence state: reduced uranium is usually much less mobile.
This research showed that framboidal pyrite may initially serve as a "sink" for uranium, where the contaminant may be sequestered under reducing conditions. Framboidal pyrites may also serve as long-term sources of contaminant uranium under conditions of slow oxidation. More studies are planned to better understand the reduction and the oxidation processes in the naturally bioreduced sediment. Clearly not all pyrites, framboidal or otherwise, contain high uranium, so understanding what controls uranium uptake in pyrite is a crucial next step.
More information: Qafoku N, RK Kukkadapu, JP McKinley, BW Arey, SD Kelly, CM Wang, CT Resch, and PE Long. 2009. "Uranium in framboidal pyrite from a naturally bioreduced alluvial sediment." Environmental Science & Technology 43(22):8528-8534; doi:10.1021/es9017333
Provided by Pacific Northwest National Laboratory (news : web)