Scientists find missing puzzle piece of powerful DNA repair complex
Researchers at The Scripps Research Institute have found, crystallized, and biologically characterized a poorly defined component of a key molecular complex that helps people to avoid cancer, but that also helps cancer cells resist chemotherapy.
The research was published in the October 2, 2009 issue of the journal Cell.
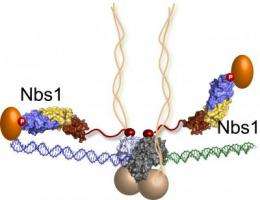
This biological machine—the Mre11-Rad50-Nbs1 (MRN) complex—senses and repairs serious forms of DNA damage, the kind that occur when both strands of the DNA helix are severed. In the new study, the scientists show how the Nbs1 component—the last major component of the complex to be described—acts like flexible arms on an octopus, reaching out and grabbing molecules needed to help in the active repair of these double-strand breaks.
Scientists believe such breaks (DNA double strand breaks, or DSBs) occur normally about 10 times a day per cell, due to normal DNA processes including replication as well as hazards such as sun exposure, etc. If these breaks are not fixed, dangerous chromosomal rearrangements occur that can lead to cancer.
"Major goals of cancer research over the past decade have been to identify the molecular underpinnings of cancer, and to provide the foundation for development of novel approaches to intervention," says Scripps Research Professor John Tainer, Ph.D., who led the research with Scripps Research Professor Paul Russell, Ph.D. "A major advance has been identification of the MRN complex as a critical suppressor of cancer development, and we now have the final piece of the picture of how cells fix a severe type of DNA damage that can lead to cancer."
But while the MRN complex is needed to keep a cell healthy, it can also unfortunately repair diseased cells that therapies are targeting for destruction. For example, a substantial proportion of chemotherapy treatments are designed to induce these double-strand breaks, which can then be repaired by the MRN complex.
"The new study suggests new ways of thinking about how to disarm this complex and improve cancer treatment," says Russell.
First Responder
The new research is the result of a long-standing collaborative effort between the structural biology laboratory headed by Tainer and the yeast genetics laboratory headed by Russell. Both laboratories have over the years helped uncover the workings of the MRN complex, which is known as the first responder to any double-strand break.
MRN's role in DNA repair is similar to a doctor's in fixing a broken leg. With a broken leg, a doctor will grab both ends of the leg bone, align them, and place them in a cast to hold them steady while the leg heals; the physician will also tell the patient not to walk on the leg until it has mended. Similarly, MRN recognizes the break in the helix, pulls the strands together, initiates homologous recombination repair of the DNA lesion, while also sending a beacon telling the cell to stop dividing until the damage is repaired.
The scientists have determined that Mre11-Rad50 proteins of the MRN complex are the first to sense double-strand breaks. The Nbs1 component then leaps into action, activating an alarm telling the cell to shut down division while coordinating activities needed to repair the break. Once activated, this complex makes use of other proteins in the cell to help send those signals and physically repair the damage. The Russell laboratory discovered one of those proteins, Ctp1, in 2007. Ctp1, known as CtIP in humans, goes directly to the site of the break and starts repairing it. Last year, both laboratories uncovered the fact that Mre11's DNA cutting activity, acting like a surgeon's scalpel, was essential for homologous recombination repair, a critical type of double-strand DNA repair.
"The MRN complex has been found in cells throughout evolution and is a key component to maintaining the integrity of DNA in all organisms," says Gerald Dodson, Ph.D., a research associate in the Russell lab and co-first author of the paper. "But it can't do repair of these breaks all alone. It needs assistance from other molecules."
While the two labs have been able to crystallize Mre11 and Rad50, and thus determine how they function, the scientists hadn't been able to do the same for Nbs1—until this study.
"The structural biology of Nbs1 was missing," says Scott Williams, Ph.D., a research associate in the Tainer lab and co-first author of the study. "We had no snapshots of what the protein looked like, so we were in the dark about how it worked, or how it can be catastrophically inactivated in human disease."
The Long Arm of MRN
In the new study, however, the scientists surmounted that challenge, shedding light on Nbs1.
The scientists found that Nbs1 is akin to an octopus arm. It is a flexible tether that extends from the octopus "head"—Mre11-Rad50—to the proteins needed to sense a break, tell the cell to cease activity, and repair the break. The researchers gained this new insight by obtaining a crystal form of Nbs1 linked to Ctp1 (a difficult technical challenge), solving its structure, and seeing that this arm could bring Ctp1 to the site of damage. The other end of the Nbs1 arm is believed to latch onto a "checkpoint" kinase that amplifies MRN's signal to stop cell division.
The importance of the Nbs1 protein was discovered through study of patients with Nijmegen breakage syndrome, a rare disorder caused by a mutation in the Nbs1 gene, according to Dodson. These patients are very sensitive to radiation and are at much greater risk than normal to developing certain cancers, including lymphomas. "In these patients, the Nbs1 tether is split in half, and we could visualize how these mutations are acting in these patients," says Williams.
Paradoxically, inducing double-strand breaks is a good way to kill cancer cells, the researchers say. "These cells are replicating much faster than normal cells, so their DNA repair systems are their Achilles heel," says Williams. "Future effective treatments might disable these repair systems, and our structural biology findings now sets up a scaffold to understand how to target these systems in order to knock them out."
Source: The Scripps Research Institute (news : web)