Growing geodesic carbon nanodomes
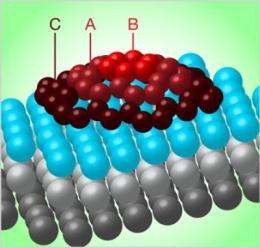
Researchers analyzing the assembly of graphene (sheets of carbon only one atom thick) on a surface of iridium have found that the sheets grow by first forming tiny carbon domes. The discovery offers new insight into the growth of graphene layers and points the way to possible methods for assembling components of graphene-based computer circuits.
Paolo Lacovig, Monica Pozzo, Dario Alfč, Paolo Vilmercati, Alessandro Baraldi, and Silvano Lizzit at institutions in Italy, the UK and USA report their discovery in a paper appearing October 12 in the journal Physical Review Letters.
The researchers' spectroscopic study suggests that graphene grows in the form of tiny islands built of concentric rings of carbon atoms. The islands are strongly bonded to the iridium surface at their perimeters, but are not bonded to the iridium at their centers, which causes them to bulge upward in the middle to form minuscule geodesic domes. By adjusting the conditions as the carbon is deposited on the iridium, the researchers could vary the size of the carbon domes from a few nanometers to hundreds of nanometers across.
Investigating the formation of graphene nanodomes helps physicists to understand and control the production of graphene sheets. In combination with methods for adjusting the conductivity of graphene and related materials, physicists hope to replace electronics made of silicon and metal with tiny, efficient carbon-based chips.
Jorge Sofo and Renee Diehl (Penn State University) highlight the graphene nanodome research in a Viewpoint in the October 12 issue of Physics.
More information: Growth of Dome-Shaped Carbon Nanoislands on Ir(111): The Intermediate between Carbidic Clusters and Quasi-Free-Standing Graphene, Paolo Lacovig, Monica Pozzo, Dario Alfč, Paolo Vilmercati, Alessandro Baraldi, and Silvano Lizzit, Phys. Rev. Lett. 103, 166101 (2009) - Published October 12, 2009, Download PDF (free)
Source: American Physical Society


















