Researchers First to 'See' Reactive Oxygen Species in Vital Enzyme
(PhysOrg.com) -- Using two simultaneous light-based probing techniques at the U.S. Department of Energy's (DOE) Brookhaven National Laboratory, a team of researchers has illuminated important details about a class of enzymes involved in everything from photosynthesis to the regulation of biological clocks.
The interdisciplinary team has a broad interest in flavoproteins, which were first discovered in the 1930s and derive from riboflavin, or vitamin B2. These proteins are now known to catalyze a wide range of biochemical reactions, including those that use molecular oxygen (O2) to help convert food into energy in animals, plants, fungi, and in some types of bacteria - a process known as oxygen activation.
Although scientists have determined more than 1,200 crystal structures of flavoproteins, they've been blind to exactly what oxygen activation looks like within these enzymes. Specifically, researchers have been unable to determine the structure of the flavoprotein's reactive oxygen intermediate, a molecular complex that often forms halfway through important biochemical reactions. These intermediates possess high chemical potential energy, which is necessary to complete many critical but difficult-to-catalyze reactions in biology. Such intermediates typically have a lifetime of only a few milliseconds and are therefore very hard to observe using traditional synchrotron methods.
"Flavoproteins represent one of only a handful of ways that nature activates molecular oxygen, a process that's important for all life on the planet," said Brookhaven biophysicist Allen Orville. "We've determined structures of some oxygen intermediates involved in several important enzymes that assist in this process. But no one has ever seen an oxygen intermediate attached to the flavin. Until now."
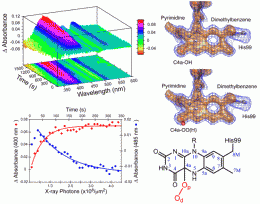
As reported in the January 9, 2009, online edition of Biochemistry, Orville and colleagues from Georgia State University, Georgia Institute of Technology, and the University of Miami have used a new facility at Brookhaven's National Synchrotron Light Source (NSLS) to identify two possible oxygen intermediates in the flavoprotein, choline oxidase.
The researchers accomplished their work by combining two popular synchrotron techniques - x-ray diffraction and optical absorption spectroscopy - into one setup. By shining beams of powerful x-rays and visible light on the same region of the crystallized flavoprotein, two different but complementary sets of information are received. This allows the scientists to correlate the electronic structure of the enzyme - which gives details about chemical activities - with its three-dimensional atomic structure.
"The ability to collect multiple types of data from the same sample at the same time is a unique opportunity," Orville said. "It takes less time and it means you never have to move the sample and risk altering it in any way. It also removes many potential ambiguities that either technique alone cannot resolve."
To stabilize the flavoprotein intermediate, the researchers kept it at an extremely low temperature - about -280 degrees Fahrenheit. When exposed to the x-rays, the cold flavoprotein rapidly accepts electrons liberated in the sample by the x-ray beam. This starts the enzyme reaction, which progresses a bit further and then becomes trapped in its reactive intermediate state. Using the combined data, the group identified two possible intermediate structures. Further experiments will help determine which is the true intermediate.
Orville is installing additional complementary techniques at the NSLS. Planning also is underway for several beamlines with multiple complementary techniques at the National Synchrotron Light Source II, a new, proposed Brookhaven facility that will produce x-rays up to 10,000 times brighter than those at the NSLS. The hope is to provide a means for researchers to simultaneously obtain three or four different types of data from one sample.
Source: Brookhaven National Laboratory