Researchers engineer pancreatic cell transplants to evade immune response
In a finding that could significantly influence the way type 1 diabetes is treated, researchers at Albert Einstein College of Medicine of Yeshiva University have developed a technique for transplanting insulin-producing pancreatic cells that causes only a minimal immune response in recipients.
At present, cell transplantation therapy is limited because transplant recipients are forced to take powerful immunosuppressant medications that have toxic side effects and raise the risk of infection. This advance in mice, described in the online version of Gene Therapy, could pave the way for routine use of cell transplants as a therapy for type 1 diabetes in humans.
Type 1 diabetes is an incurable autoimmune disease in which the immune system mistakenly destroys the body's own pancreatic beta cells. Beta cells produce insulin, which breaks down sugar, or glucose, for use by the body. Without these cells, too much glucose builds up in the blood. High blood glucose levels damage cells and can eventually lead to complications such as heart disease, kidney disease, blindness, and premature death.
Type 1 diabetes affects up to 2.4 million Americans and can develop at any age, though it typically appears during childhood or adolescence. People with type 1 diabetes must closely monitor their blood glucose levels and take daily insulin injections for life.
A promising alternative to insulin injections is cellular transplantation, in which beta cells are harvested from cadavers and injected into the bloodstream of patients with diabetes; the new cells replace the recipients' destroyed pancreatic beta cells. Although such transplants can control type 1 diabetes, recipients must take immunosuppressant medications in order to prevent rejection of these beta foreign cells. "Ultimately, even with immunosuppressive therapy, most of these individuals end up rejecting the transplanted cells," says the study's principal investigator, Harris Goldstein, M.D., professor of pediatrics and of microbiology & immunology at Einstein.
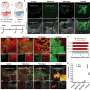
In this study, Dr. Goldstein and his colleagues devised a way to make foreign beta cells invisible to a transplant recipient's immune system, dramatically protecting them from rejection. They did so by harnessing the innate ability of adenoviruses to evade the body's immune surveillance system. (Adenoviruses infect tissues that line the respiratory tract, eyes, intestines, and urinary tract). After infecting cells, adenoviruses produce proteins that prevent the cells from signaling the immune system that they have been infected and should be destroyed. The viruses also produce proteins that can turn off a cell's built-in self-destruct mechanism, which is usually triggered when something disturbs a cell's internal functions.
The researchers began with a special line of insulin-producing beta cells, developed at Einstein, that were harvested from mice. When injected into diabetic mice, these foreign cells can restore normal glucose control, but only temporarily. The transplanted cells are soon destroyed by the mouse's immune system and glucose levels begin to rise, returning to pre-transplant disease levels.
Dr. Goldstein and his colleagues genetically engineered these beta cells to include three adenoviral genes responsible for making immunosuppressive proteins. Diabetic mice that received these engineered foreign beta cells maintained normal glucose control for up to three months. In contrast, a control group of diabetic mice that received the regular foreign beta cells exhibited normal glucose control for just a few days.
"Clearly, the three proteins were not optimal, because ultimately the cells did get rejected," says Dr. Goldstein. "We are now looking at other viral genes that also contribute to immune suppression and are trying to identify the best gene combination to use."
Dr. Goldstein views the current experiment as a proof of concept. "We were able to demonstrate that genetically engineered beta cells can be made highly resistant to rejection and can basically correct diabetes. This technique could conceivably be applied to protect any type of cellular transplant from rejection."
However, pancreatic cell transplantation could not help treat patients with type 2 diabetes. In this form of the disease, patients have fully functional beta cells but cells throughout their body become resistant to insulin.
Source: Albert Einstein College of Medicine