Researchers create new class of fluorescent dyes to detect reactive oxygen species in vivo
Researchers have created a new family of fluorescent probes called hydrocyanines that can be used to detect and measure the presence of reactive oxygen species. Reactive oxygen species are highly reactive metabolites of oxygen that have been implicated in a variety of inflammatory diseases, including cancer and atherosclerosis.
"We've shown that the hydrocyanines we developed are able to detect the reactive oxygen species, superoxide and the hydroxide radical, in living cells, tissue samples, and for the first time, in vivo," said Niren Murthy, assistant professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University.
Details of the hydrocyanine synthesis process and experimental results showing the ability of the dyes to detect reactive oxygen species in cells, tissues and mouse models were reported on December 8 in the online version of the journal Angewandte Chemie International Edition. This research is supported by the National Institutes of Health and the National Science Foundation.
The researchers have created six hydrocyanine dyes to date – hydro-Cy3, hydro-Cy5, hydro-Cy7, hydro-IR-676, hydro-IR-783 and hydro-ICG – but say that there are potentially 40 probes that could be created. The dyes vary in their ability to detect intracellular or extracellular reactive oxygen species and by their emission wavelength – from 560 to 830 nanometers.
Fluorescing at higher wavelengths allows the hydrocyanine dyes to be used for deep tissue imaging in vivo, a capability that dihydroethidium (DHE), the current "gold standard" for imaging reactive oxygen species, does not have. The dyes also have other advantages over DHE.
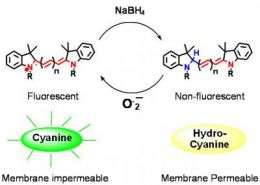
"When DHE comes into contact with reactive oxygen species, it oxidizes into ethidium bromide, a common mutagen, which means it's toxic and can't be injected inside the body," explained Murthy. "DHE also auto-oxidizes in the presence of aqueous solutions, which creates high levels of background fluorescence and interferes with reactive oxygen species measurements."
Hydrocyanines are also simple and quick to synthesize, according to Coulter Department postdoctoral fellow Kousik Kundu. Sodium borohydride is added to commercially available cyanine dyes and the solvent is removed – the one-step process takes less than five minutes.
W. Robert Taylor, a professor in the Coulter Department and Emory's Division of Cardiology, and Emory postdoctoral fellow Sarah Knight, tested the ability of the dyes to detect reactive oxygen species inside of cells and animals.
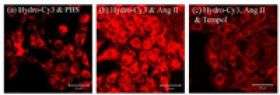
For their first experiment, they tested the ability of hydro-Cy3, which has an emission wavelength of 560 nanometers, to detect reactive oxygen species production in the aortic smooth muscle cells of rats. They incubated the cells with hydro-Cy3 and angiotensin II, which is a stimulator of reactive oxygen species that is implicated in the development of atherosclerosis and hypertension.
Results showed that cells incubated with angiotensin II and hydro-Cy3 displayed intense intracellular fluorescence, whereas control cells incubated with hydro-Cy3 and phosphate buffer saline displayed significantly lower fluorescence. When they introduced TEMPOL, a molecule that intercepts the reactive oxygen species so that they cannot interact, the cells treated with angiotensin II and hydro-Cy3 displayed a dramatic decrease in fluorescence.
"This test demonstrated that the cellular fluorescence was due to intracellular reactive oxygen species production," said Murthy. "What was even more exciting was that we saw that once the hydrocyanine dye was oxidized, it stayed in the cell and the fluorescence was not extinguished by cellular metabolism, which is what happens with DHE."
The researchers also investigated the ability of hydro-Cy3 to image reactive oxygen species production in live mouse aorta tissue, which exhibit a physiological environment that closely resembles in vivo conditions. Explants were incubated with hydro-Cy3 and either lipopolysaccharide endotoxin (LPS), an inflammatory molecule that binds to aortic cells and causes reactive oxygen species to be produced, or the control saline solution.
Samples treated with hydro-Cy3 and LPS showed fluorescence intensity almost four times greater than explants treated with hydro-Cy3 and saline. Once more, adding TEMPOL to the sample with hydro-Cy3 and LPS decreased the fluorescence to a level comparable to the control saline explants.
After the successful cell culture and tissue experiments, the researchers progressed to in vivo mouse imaging studies. Hydro-Cy7 was selected for the in vivo tests because of its higher emission wavelength of 760 nanometers. LPS-treated mice showed twofold greater fluorescence intensity in the abdominal area than those treated with saline.
"Given their ability to detect reactive oxygen species in living cells, tissue samples and in vivo, we believe these dyes will enhance the ability of researchers to measure reactive oxygen species," noted Murthy.
The researchers' ultimate goal, though, is to use the dyes in clinical applications.
"We want to use these hydrocyanine dyes to detect overproduction of reactive oxygen species at an early stage inside the body so that we can identify patients who are more likely to suffer from these inflammatory diseases," added Murthy.
Source: Georgia Institute of Technology