Researchers reshape the future of drug discovery
Scientists in Leeds have devised a new way to create the next generation of man-made molecules in a breakthrough that could revolutionise drug development.
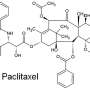
Creating new drugs to combat disease and illness requires the completion of a complex 3D jigsaw. The shape of the drug must be right to allow it to bind to a specific disease-related protein and to work effectively, and this shape is determined by the core framework of the molecule.
Now a team from the Astbury Centre for Structural Molecular Biology at the University of Leeds has developed a new approach which allows the creation of molecules with an extraordinarily wide range of molecular frameworks and, hence, shapes. The new molecules are likely to have a wide range of biological functions, which means they could be valuable starting points for the discovery of new drugs.
Says lead researcher Professor Adam Nelson of the University's School of Chemistry: "Nature has created hundreds of thousands of molecules that have different frameworks and biological purposes, but in the global pursuit of new drugs, chemists from around the world are racing to create new molecules with functions not seen in nature."
The newly created molecules are being shared with colleagues in the Faculties of Biological Sciences and Medicine and Health to see if specific new molecular frameworks match the requirements of their own research.
Of the 30 million or so synthetic molecules made throughout the history of organic chemistry, many are based on an extremely small number of core frameworks, with the main differences being the groups attached at the periphery. "Making collections of similar molecules is great for optimising a biological property," says Professor Nelson, "but to put it simply, if researchers need a cube-shaped molecule to target a particular protein, they may well find that they can only choose from libraries stocked with millions of sphere-shaped ones."
Co-researcher Dr Stuart Warriner added: "Making molecules is a bit like making something using lego bricks. Up until now we've only really become good at making, say, the equivalent of a lego car or train. There might be 30 million synthetic molecules registered, but there's probably several million of these that are the equivalent of lego cars – they may have different wheels and wing mirrors, but their fundamental shape is essentially the same. We've not really scratched the surface of the possible structures that could be made. This lack of variety in the core shape of molecules may well limit the range of proteins that medicinal chemists can target."
The Leeds approach makes use of 'metathesis', a reaction that won the 2005 Nobel Prize in Chemistry.
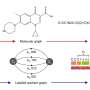
Explains Professor Nelson: "We take simple building blocks, a bit like the amino acids that make up peptides, and we assemble them in different sequences using three simple reactions to link them together in a chain. The key difference is that we then add the catalyst which initiates a 'scaffold reprogramming reaction', which ripples down the chemical chain and restitches the molecule together in a completely different way each time.
"It's a bit like a molecular square dance, where atoms in the molecule swap partners - and the exciting thing is that we can change the building blocks again and again in different combinations as a really powerful way to vary the core frameworks that result. The potential of this process is enormous," he says.
The team from Leeds have used their approach to prepare molecules with 84 distinct molecular frameworks – and about two-thirds of the frameworks are unprecedented in the history of organic chemistry. The work is a huge leap forward from landmark research reported in 2003, which resulted in the creation of six frameworks in a single process. It is also a significant improvement on more recent research in which around 30 frameworks were created using a complex combination of different reactions.
The team has deliberately chosen to prepare molecules with structural features that are similar to those found in natural products: "For example we know that putting oxygen atoms on every other carbon atom is something that frequently occurs in nature and has evolved for a useful purpose" says Professor Nelson. "We're not aiming to improve on existing natural products or drugs - we want to create molecules with functions that nature's not got round to making yet, or something that would only evolve naturally with new selection pressures that would make it beneficial for the organism."
Work has already begun across campus to screen the molecules, which are already yielding "promising" results. The team are considering patenting molecules with novel biological functions.
Source: University of Leeds