Scripps Research scientists define structure of important neurological receptor
Scientists from The Scripps Research Institute have determined the structure of an adenosine receptor that plays a critical role in a number of important physiological processes including pain, breathing, and heart function. The findings could lead to the development of a new class of therapeutics for treating numerous neurological disorders, including Parkinson's and Huntington disease.
"We are developing a robust platform for studying human G protein-coupled receptor structure and function," Stevens said. "This work lays a strong foundation for understanding drug-receptor interactions. We expect to continue our work and develop a deep understanding as to how drugs interact with the broader class. The findings—and our future research—could one day lead to the development of a novel class of therapeutics with improved pharmaceutical properties."
The new study defined the structure of the human A2A adenosine receptor—sometimes referred to as the "caffeine receptor"—which falls in the larger family of G protein-coupled receptors (GPCR).
"Last year, we determined the structure of the β2-adrenergic G protein-coupled receptor with multiple ligands," said Raymond Stevens, a Scripps Research scientist and professor. "The big question then was—is it going to be another 10 years until we get the next new receptor? The answer is 'no.' It has taken less than a year to determine this new structure. Our expectation is that even more will come out in the next few years."
Because of the importance of GPCRs to health and medicine and previous lack of knowledge about their structure, the Stevens lab's 2007 research solved the structure of the β2-adrenergic G protein-coupled receptor and was selected as one of the top 10 breakthroughs of the year by Science magazine (see http://www.sciencemag.org/cgi/content/full/318/5858/1844a).
"The National Institutes of Health supports programs specifically designed to develop technology to elucidate the structures of membrane proteins such as G-protein coupled receptors, which are critical for almost all aspects of health and disease," said Jeremy M. Berg, director of the NIH's National Institute of General Medical Sciences. "The recent successes with the new methods foreshadow exciting future advances in determining the structures of other medically important proteins."
In the new study, the Stevens laboratory worked with the IJzerman laboratory at the Leiden/Amsterdam Center for Drug Research in The Netherlands, to illuminate the A2A adenosine receptor. This receptor is blocked by methylxanthines like caffeine, which prevents the binding of other naturally occurring ligands. Interestingly, there is evidence that coffee drinkers have a lower risk of Parkinson's disease.
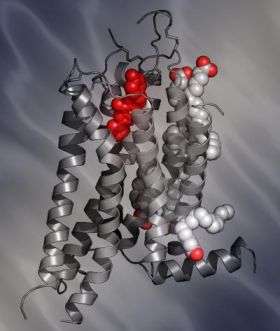
Because membrane proteins like adenosine receptors have been notoriously difficult to crystallize—a key step in determining the structure of a molecule through the technique of x-ray crystallography—the scientists bound the A2A adenosine receptor to a high-affinity antagonist, ZM241385. ZM241385, which had been developed as a potential drug to combat Parkinson's disease, stabilizes the receptor.
With the two molecules bound together, the scientists were able to obtain crystals of the complex, and determine its structure.
A Big Surprise
The crystallographic model of the A2A receptor bound to ZM241385 reveals features distinct from previously reported GPCR structures.
With over one thousand members, G protein-coupled receptors are one of the most diverse protein families in the human genome. They transduce or convert extracellular stimuli into intracellular signals through a number of signaling pathways including neurotransmitters, light, hormones, lipids, and proteins. Because of their diverse signaling pathways, approximately one third, and perhaps as many as half, of currently marketed drugs are designed to target these receptors.
Extracellular adenosine plays an important role in physiology and initiates most of its effects through the activation of four GPCR subtypes, A1, A2A, A2B, and A3. Each of these four receptors plays an essential role in responding to adenosine in the central nervous system in pain regulation, cerebral blood flow, basal ganglia functions, respiration, and sleep.
In the new study, the A2A adenosine-ligand bound structure suggests that there is no general receptor binding pocket conserved across the adenosine receptor family. Rather, the pocket itself can vary in position and orientation, yielding more opportunity for receptor diversity and ligand selectivity.
"A big surprise for us seeing the structure was that the ligand was in an extended conformation and pointed perpendicular to the membrane, interacting with the extracellular loops," Stevens said.
This feature can be seen as the rationale for A2A receptor selectivity and may help in the design of new chemical entities with increased selectivity for this important drug target.
Source: Scripps Research Institute