Scientists create world's thinnest balloon, just 1 atom thick
(PhysOrg.com) -- Using a lump of graphite, a piece of Scotch tape and a silicon wafer, Cornell researchers have created a balloonlike membrane that is just one atom thick -- but strong enough to contain gases under several atmospheres of pressure without popping.
And unlike your average party balloon -- or even a thick, sturdy glass container -- the membrane is ultra-strong, leak-proof and impermeable to even nimble helium atoms.
The research, by former Cornell graduate student Scott Bunch (now an assistant professor at the University of Colorado), Cornell professor of physics Paul McEuen and Cornell colleagues, could lead to a variety of new technologies -- from novel ways to image biological materials in solution to techniques for studying the movement of atoms or ions through microscopic holes.
The work was conducted at the National Science Foundation-supported Cornell Center for Materials Research and published in a recent issue of the journal Nano Letters.
Graphene, a form of carbon atoms in a plane one atom thick, is the strongest material in the world, with tight covalent bonds in two dimensions that hold it together even as the thinnest possible membrane. It's also a semimetal, meaning it conducts electricity but changes conductivity with changes in its electrostatic environment.
Scientists discovered several years ago that isolating graphene sheets is as simple as sticking Scotch tape to pure graphite, then peeling it back and re-sticking it to a silicone dioxide wafer. Peeled back from the wafer, the tape leaves a residue of graphite anywhere from one to a dozen layers thick -- and from there researchers can easily identify areas of single-layer-thick graphene.
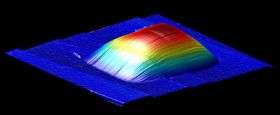
To test the material's elasticity, the Cornell team deposited graphene on a wafer etched with holes, trapping gas inside graphene-sealed microchambers. They then created a pressure differential between the gas inside and outside the microchamber. With a tapping atomic force microscope, which measures the amount of deflecting force a tiny cantilever experiences as it scans nanometers over the membrane's surface, the researchers watched the graphene as it bulged in or out in response to pressure changes up to several atmospheres without breaking.
They also turned the membrane into a tiny drum, measuring its oscillation frequency at different pressures. They found that helium, the second-smallest element (and the smallest testable gas, since hydrogen atoms pair up as a gas), stays trapped behind a wall of graphene -- again, even under several atmospheres of pressure.
"When you work the numbers, you would expect that nothing would go through, so it's not a scientific surprise," said McEuen. "But it does tell you that the membrane is perfect" -- since even an atom-sized hole would allow the helium to escape easily.
Such a membrane could have all kinds of uses, he added. It could form a barrier in an aquarium-like setup, for example, allowing scientists to image biological materials in solution through a nearly invisible wall without subjecting the microscope to the wet environment. Or, researchers could poke atomic-sized holes in the membrane and use the system to study how single atoms or ions pass through the opening.
"This could serve as sort of an artificial analog of an ion channel in biology," McEuen said -- or as a way to measure the properties of an atom by observing its effect on the membrane.
"You're tying a macroscopic system to the properties of a single atom," he said, "and that gives opportunities for all kinds of single atom sensors."
The paper's co-authors are Cornell physics graduate students Arend van der Zande and Jonathan Alden; postdoctoral researcher Scott Verbridge; and professors Jeevak Parpia and Harold Craighead.
Provided by Cornell University