Protein thought to promote cancer instead functions as a tumor suppressor

A protein previously thought to promote colorectal cancer instead suppresses the growth of human cancer cells in culture, researchers at UT Southwestern Medical Center have found.
"This finding reshapes a fundamental model of how colorectal cancer arises," said Dr. Lawrence Lum, assistant professor of cell biology at UT Southwestern and senior author of the study, which appears online today and in a future issue of the Proceedings of the National Academy of Sciences.
Approximately 90 percent of colorectal cancers are caused by a biochemical malfunction that arises from mutations in a gene that activates a gene called TCF7L2, Dr. Lum said. As a result, TCF7L2 has been suspected of helping to trigger colorectal cancer.
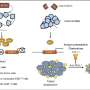
In the current study, the researchers used a novel genetic screening approach known as RNAi mediated interference, or RNAi, to identify genes that contributed to this malfunction.
The researchers employed more than 80,000 small snippets of chemically synthesized RNAs (ribonucleic acids) known as "small interfering RNAs" or siRNAs, that are each capable of inactivating a specific gene. The researchers mixed these siRNAs with specially engineered human cancer cells that glowed when the cancer-causing malfunction is activated. When an siRNA made a cell glow, the researchers were able to flag the gene as a candidate cancer gene.
As the researchers examined the genes of interest more closely, they unexpectedly found that a gene called TCF7L2, which had been thought to boost malignant cell growth, instead suppressed it. When the gene was inactivated, human colorectal cancer cells grew more rapidly in culture and emitted a stronger glow.
"The function of TCF7L2 in cancer was previously determined from studies in animals but no one has genetically tested its role in human colorectal cancer cells before," said Dr. Lum, who is a Virginia Murchison Linthicum Scholar in Medical Research. "Prior to the advent of RNAi technology, this was very difficult to do in human cultured cells."
The next step is to understand more fully all the steps in the biochemical pathway involved in controlling the action of TCF7L2. This knowledge could then be used to identify new therapeutic targets for treating colorectal cancer. Therapeutic strategies derived from such studies may also be useful in treating type II diabetes, for which risk is strongly associated with mutations in TCF7L2, Dr. Lum said.
Dr. Lum noted that the findings about TCF7L2 show the effectiveness of this high throughput screening technique, which can quickly and systematically check many thousands of genes.
The success of this relatively new screening method for identifying candidate cancer genes demonstrates its usefulness to understanding human disease, Dr. Lum said.
"It's a way to analyze human gene action directly in human tissue at a genome-scale," he said.
Source: UT Southwestern Medical Center