Surprising graphene: Honing in on graphene electronics with infrared synchrotron radiation
Graphene is the two-dimensional crystalline form of carbon: a single layer of carbon atoms arranged in hexagons, like a sheet of chicken wire with an atom at each nexus. As free-standing objects, such two-dimensional crystals were believed impossible to create -- even to exist -- until physicists at the University of Manchester actually made graphene in 2004.
Now researchers at the Department of Energy's Advanced Light Source (ALS), from DOE's Lawrence Berkeley National Laboratory and the University of California at San Diego (UCSD), have measured the extraordinary properties of graphene with an accuracy never before achieved.
The results confirm many of the strangest features of the unusual material but also reveal significant departures from theoretical predictions. And they point the way to novel practical applications, such as tunable optical modulators for communications and other nanoscale electronics.
The studies were performed by Zhiqiang Li, an ALS Doctoral Fellow from Dimitri Basov's laboratory at UCSD, working with colleagues at Columbia University in New York and the National High Magnetic Field Laboratory in Florida, and with Berkeley Lab's Michael Martin, who manages the Fourier Transform Infrared beamline 1.4.4 at the ALS. The researchers report their findings in the June issue of the journal Nature Physics.
Graphene's promising electronic properties
The familiar pencil-lead form of carbon, graphite, consists of layers of carbon atoms tightly bonded in the plane but only loosely bonded between planes; because the layers move easily over one another, graphite is a good lubricant. In fact these graphite layers are graphene, although they had never been observed in isolation before 2004. Once demonstrated, research immediately took off, inspired by the material's unexpected electronic properties. Experiments continue unabated.
"Graphene's unusual electronic properties arise from the fact that the carbon atom has four electrons, three of which are tied up in bonding with its neighbors," says Li. "But the unbound fourth electrons are in orbitals extending vertically above and below the plane, and the hybridization of these spreads across the whole graphene sheet."
As a crystal, two-dimensional graphene is quite dissimilar from three-dimensional materials such as silicon. In semiconductors and other materials, charge carriers (electrons and oppositely charged "holes") interact with the periodic field of the atomic lattice to form quasiparticles (excitations that act like actual particles). But quasiparticles in graphene do not look anything like an ordinary semiconductor's.
The energy of quasiparticles in a solid depends on their momentum, a relationship described by energy bands. In a typical 3-D semiconductor, the energy bands are "parabolic" -- a graph of the lower, filled valence band resembles a stalagmite, more or less flat on top, while the upper, empty conduction band is its opposite, a stalactite, more or less flat on the bottom; between them is the open band gap, representing the amount of energy it takes to boost an electron from the valence band to the conduction band.
Unlike in ordinary semiconductors, however, graphs of the valence band and the conduction band in graphene are smooth-sided cones that meet at a point, called the Dirac point. These plot the energy-momentum relationships of quasiparticles behaving as if they were massless electrons, so-called Dirac fermions, which travel at a constant speed -- a small but noteworthy fraction of the speed of light.
One interesting consequence of this unique band structure is that the electrons in graphene are "sort of free," Li says. Unlike electrons in other materials, the electrons in graphene move ballistically -- without collisions -- over great distances, even at room temperature. As a result, the ability of the electrons in graphene to conduct electrical current is 10 to 100 times greater than those in a normal semiconductor like silicon at room temperature. This makes graphene a very promising candidate for future electronic applications.
Says Li, "By applying a gate voltage to graphene which has been integrated in a gated device, one can continually control the carrier density by varying the voltage, and thus the conductivity." It's this phenomenon that gives rise to graphene's practical promise.
An unusual experiment
Because graphene is tricky to make and even trickier to handle, most experiments have been performed not on free-standing monolayers but on two types of graphene samples: exfoliated graphene, deposited on a silicon-oxide/silicon substrate, and epitaxial graphene, a layer of carbon atoms chemically deposited (and chemically bonded to) a substrate of silicon carbide.
"Epitaxial graphene and exfoliated graphene are very distinct," says Li. Much of the synchrotron experimental work on epitaxial graphene has employed ARPES, angle-resolved photoemission spectroscopy. "In our work, we investigated exfoliated graphene by employing infrared spectromicroscopy."
Li explains that infrared measurements can probe the dynamical properties of quasiparticles over a wide energy range, and therefore can provide some of the most interesting information about the electronic properties of a material, "such as electron lifetime and interactions between electrons," he says. "Such measurements have not been performed on exfoliated graphene before, because it is extremely difficult to measure the absorption of light in a single monolayer of graphene."
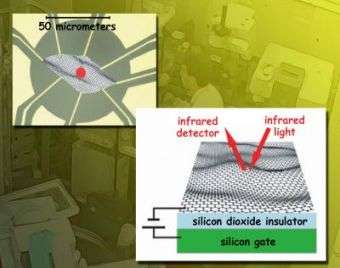
To accurately measure how graphene's infrared absorption responds to varying the gate voltages, the researchers first needed samples of exfoliated graphene attached to electrodes. They used flakes of single-atomic-layer graphene 50 micrometers (millionths of a meter) square. The samples were laid on top of (but not chemically bonded to) an insulating layer of silicon oxide and an underlying layer of pure silicon. This transparent substrate acted as the gate electrode. The whole system was cooled to 45 kelvins (379 degrees Fahrenheit below zero).
"To measure the absorption of infrared light by neutral graphene and by electrostatically doped graphene" -- that is, not chemically doped, but rather "doped" by the gate voltage -- "we needed the intensity and tight focus of a beam of synchrotron radiation operating at infrared frequencies," says Michael Martin. "The ALS IR beam, less than 10 micrometers across, was positioned at various points across the sample, which allowed us to directly measure transmission and reflectance and obtain the sample's optical conductivity."
The term "optical" in optical conductivity refers to the high frequency of light, as opposed to the very low frequency of household alternating current (ac). The researchers induced optical-frequency ac in the graphene sample with the infrared beam and manipulated the voltage via the electrodes. The changes in voltage changed the conductivity and carrier density in the sample, and directly affected its ability to transmit or reflect light. Indeed, the researchers found that the reflectance and transmission of graphene can be dramatically tuned by applied gate voltages.
"In a normal electronic system such as a semiconductor, the Fermi energy" -- the energy of the carriers' highest occupied quantum state when the temperature is absolute zero -- "is proportional to the density of the carriers," Li says. "But in a system of 2-D Dirac fermions, the Fermi energy is proportional to the square root of the carrier density."
The researchers observed this unique square-root density dependence of the Fermi energy, which verifies that electrons in graphene indeed behave like Dirac fermions. Thus many of the effects predicted for graphene by theory were confirmed in these experiments, and measured to an accuracy never before obtained.
Graphene surprises
Other results, however, revealed "many-body interactions," more complex than what had been suggested by the "single-particle" picture of graphene, which treats the carriers as an ensemble of independent particles. Theories predict that if electrons in graphene experience no interactions with one another or with the carbon atoms, then at low energy (or frequency) -- energy below twice the Fermi energy -- they will absorb hardly any light.
Instead, the researchers observed considerable absorption of infrared light in this low-energy region. "This unexpected absorption may stem from interactions between electrons and lattice vibrational modes of the carbon atoms, or from mutual interactions among electrons," says Li.
Another surprise is the speed of the electrons. As independent particles, electrons in graphene should travel at a constant speed regardless of their energy. The researchers found that, at high energies, electrons in graphene indeed move at constant speed -- but their speed increases systematically as their energy is lowered. This enigmatic behavior may be due to electron-electron interactions, predicted theoretically in 1994.
"Many-body interactions could be as simple as Coulomb interactions between electrons, or they could be more complicated," says Martin. "The data give a clear signature, but we don't completely understand them."
"Some of these effects might be related to the limitations of the samples we studied," says Li. "We'd like to do the measurements on samples suspended over a void, so as to eliminate any disorder induced by the substrate. Still, even allowing for refinements, it appears that our measurements present a challenge to the current understanding of this intriguing material."
Citation: "Dirac charge dynamics in graphene by infrared spectroscopy," by Z. Q. Li, E. A. Henriksen, Z. Jiang, Z. Hao, M. C. Martin, P. Kim, H. L. Stormer, and D. N. Basov, appears in the June issue of Nature Physics and is available online to subscribers at www.nature.com/nphys/journal/v … /ncurrent/index.html
Source: DOE/Lawrence Berkeley National Laboratory