December 20, 2007 feature
'Kind and Gentle' Molecular Machine Could Operate at Near-Equilibrium
Molecular machines – tiny machines made of molecules that do mechanical work – are usually thought to operate in a state of non-equilibrium. This makes sense, considering that macro-sized machines operate at non-equilibrium, requiring an additional force to move. On the other hand, equilibrium implies that forces cancel each other out, resulting in an unchanging system, often at rest.
But R. Dean Astumian, a Physics Professor at the University of Maine, has recently proposed a concept in which molecular machines can operate arbitrarily close to chemical equilibrium at every instant of the cycle, and still perform work at the rate of several micrometers per second against piconewton loads. The study, “Adiabatic operation of a molecular machine,” is published in a recent issue of the Proceedings of the National Academy of Sciences.
“The main significance is conceptual – it changes the way we think about molecular motors,” Astumian told PhysOrg.com. “Much emphasis has been put on the ‘non-equilibrium’ aspects of the system, but in fact this is not really important. The motion of the rings here arises due to a combination of topology that break spatial symmetry, and the slow external modulation that breaks time symmetry. It is also important to recognize that, in the molecular world, we can truly have motors that operate with nearly 100% efficiency.”
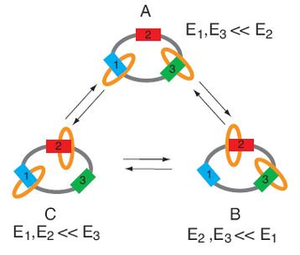
Astumian’s example of such a molecular machine is a three-ring “catenane” structure that serves as a rotating motor. The catenane, about 3 nanometers in diameter, consists of one large ring with two smaller rings linked to the large ring, like rings on a keychain. Three binding stations on the large ring provide locations where the two small rings can bind, depending on the interaction energy between ring and station.
Astumian gets the small rings to move clockwise from station to station around the large ring, a movement that results in mechanical cycling. Further, he achieves this movement without any heat gain or heat loss and without a change of entropy, but simply by thermal noise due to Brownian motion. This type of “adiabatic” system is arbitrarily close to equilibrium at every point of the cycle.
The key to making the small rings move from station to station is by periodically modulating the interaction energy. The rings will bind to the station that requires the lowest interaction energy. This modulation must be done slowly enough not to generate heat, but at a sufficient rate to produce significant work.
Slowly modulating the energy of a single molecule is challenging, and Astumian explains that it is nearly impossible to do with chemical systems. However, the net interaction energy for many molecules can be slowly modulated by very slow titrations of pH and redox potential. For example, a condition that is strongly acidic and strongly reducing corresponds with high interaction energy at station 2. Therefore, the two rings will bind at stations 1 and 3.
Then, by titrating with oxidant to reach a strongly oxidizing and strongly basic condition, the energy at station 1 is highest. So the rings now occupy stations 2 and 3. Astumian explains that the rings could switch to this state by either the ring at station 1 moving clockwise to bind at station 2, or the ring at station 3 moving counter-clockwise to bind at station 2.
However, after more modulations, the majority (about 75%) of the movements will be clockwise due to the changing energy levels at different stations. By figuring this probability, Astumian concludes that, every time the small rings move around the large ring, the system will complete one-half of a mechanical rotation.
He also mentions that there might be more creative architectures where counter-clockwise motions don’t undo the clockwise motions. He likens this mechanism to a ratcheting screwdriver used to drive a screw. When the ratchet turns counter-clockwise to reset itself, it releases so that it doesn’t undo the forward turn of the screw. Although a molecular machine would use thermal noise instead of external torque, the concept is similar. In the molecular machine, one of the small rings could be fixed to prevent counterclockwise motion.
“I would say that the biggest challenge is to arrange the molecules on a surface so that the movement can be used to do work on the outside world,” Astumian said. He added that the chemical structures have already been synthesized by David Leigh at the University of Edinburgh, as the next step in the development of the machines.
Astumian also explains that operating this system requires a fine balance between modulating slowly enough so that the system is in chemical and mechanical equilibrium at every instant, but rapidly enough to perform substantial work. While biomolecular motors are often described in “violent” terms, he hopes that this “kindler, gentler” description may be more appropriate for designing more efficient molecular machines.
“I plan to extend the theory to explain the mechanism of biological motors,” Astumian said. “While they doubtless do not operate exactly by the mechanism described for the three-ring catenane, I think the flavor of how they operate is much better characterized by this near-equilibrium description than by various biological models involving cars, judo throws, and steam engines.”
More information: Astumian, R. Dean. “Adiabatic operation of a molecular machine.” PNAS, December 11, 2007, vol. 104, no. 50, 19715-19718.
Copyright 2007 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.

















