Hatching a New Model for Biomineralization
The idea started with an eggshell and ended with a new understanding of how minerals form to build exceptionally strong structures in the bodies of humans and other organisms. Biomineralization, the process by which organisms form materials such as bones, mollusk shells, and other structures, has captured the attention of scientists for years.
Finding a way to mimic the properties of these sturdy and naturally made materials could lead to the medical engineering of replacement bone, teeth, and cartilage, as well as the development of new electronic and industrial materials. Most of the research surrounding biomineralization has looked at the multiple processes it involves and the diversity of its products. But at the NSLS, using inspiration from an egg, a team of researchers studied the earliest stages of biomineralization to find out what sets the process in motion.
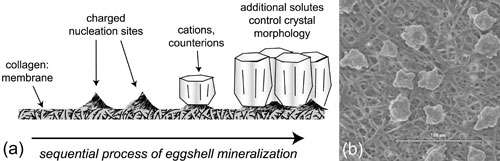
A bird’s eggshell is about a half-millimeter of layered calcium carbonate crystals, stabilized by a protein matrix. The shell forms during just about 12 hours of travel time through the bird’s oviduct, an amazing natural feat, said NSLS physicist Elaine DiMasi, one of the authors of the biomineralization study that was published in the October 3, 2006 edition of the Proceedings of the National Academy of Sciences.
“It starts as a collagen membrane and goes through a series of different fluids with different species in them, and in the end, you have this hard mineral,” she said. “We were looking for a system that would mimic some features of that eggshell.”
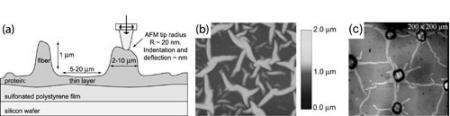
To model extracellular biomineralization, the formation of materials on the outside of the cell wall, such as in the case of egg shell formation, the research team used a self-assembled protein network with both fibronetin and elastin – major connective tissue components in multicellular organisms. These proteins were incubated on negatively charged surfaces in two forms: structurally organized fibers and regions with a thin unorganized layer of protein wedged in between them.
After exposing the system to calcium carbonate for a varying set of times, the researchers used a relatively new technique called shear modulation force microscopy (SMFM) to compare the response of the two sets of protein fibers. SMFM is an atomic force microscopy-based technique in which a cantilever with a superfine tip just 40 nanometers wide is stuck into the soft material being studied. The tip is then vibrated to measure the stiffness of the material, and thus whether or not mineralization occurred on the protein fibers.
The group found that the calcium carbonate stiffened only the organized protein fibers, without affecting the unorganized regions between them. This demonstrates that mineralization requires structural organization of the protein in order to function, DiMasi said.
“It’s exciting that there’s a demonstration of disorganized and organized proteins side by side in the same exact environment,” DiMasi said. “Any other experiment would have just inferred that an organized protein structure was necessary to nucleate, but there’s never been a comparison like this between disorganized and organized protein.”
Besides the actual results, the setup of the experiment itself, including the model system and the SMFM technique, provides valuable information for the scientific community – and not just to study eggs.
“This looks like a really good model system,” DiMasi said. “Now one could take collagen and calcium phosphate and study bone nucleation or any other number of things. It just looks like a really good platform.”
Other scientists involved in the study are Seo-Young Kwak (NSLS); Karthikeyan Subburaman, Nadine Pernodet, Shouren Ge, Vladimir Zaitsev, Xiaolan Ba, and Miriam Rafailovich (Stony Brook University); and Nan-loh Yang (City University of New York).
Source: by Kendra Snyder, Brookhaven National Laboratory