Lasers Shine Light on Chemical Reactions
Scientists at the U.S. Department of Energy’s Brookhaven National Laboratory have been using a high-resolution laser technique to learn how molecules absorb light and fall apart during photodissociation reactions — chemical decomposition reactions triggered by light. Studying the atomic-level details of such reactions allows scientists to test and refine theories of chemical reactions, and may help them in their quest to use light to control reaction outcomes.
“Despite much research in this field, there remain many unanswered details at the frontier of our understanding about chemical bond-breaking following light absorption,” said chemist Greg Hall, leader of the Brookhaven research team. A paper by Hall’s group describing new experimental and theoretical results has been identified as a “hot article” by the journal Physical Chemistry Chemical Physics, and is now available online.
The Brookhaven team was studying the photodissociation of a much-studied compound composed of one atom each of iodine, carbon, and nitrogen (ICN), which breaks into an iodine atom and a carbon-nitrogen radical upon exposure to light. One reason this molecule has attracted so much attention is because its photodissociation is a particularly simple example of a reaction that can produce the same products by way of two different paths, both initiated by the same pulse of light.
“This is an ideal opportunity to explore in a chemical reaction the effects of quantum interference — the ability of matter to act like a wave, with components that reinforce or cancel one another when combined, depending on the relative position, or phase, of the crests and valleys of the waves,” Hall said. “In the microscopic world of molecules, combining the same two waves with a different phase can change some properties of the product completely.”
In the case of ICN, the two reaction paths correspond to different excited states, or rearrangements of the electrons. When excited, the electrons no longer hold the molecule together. As the normally linear molecule falls apart, the excited electrons also make it start to bend, leading to a rotation of the CN fragment.
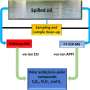
Using one laser pulse to start the reaction and another laser to probe it, the scientists can capture the details of fragment rotation after the fragments have formed but before they’ve had a chance to be disturbed by collisions with other molecules around them.
“What we actually measure is how fast and which way the fragments are going and around what axis they are rotating as they separate,” Hall said. “To do this, we measure how much of the probe laser beam is absorbed by the sample as we change its frequency (or color) by very small amounts, and compare the shapes of the absorption spectra measured with different combinations of beam directions and polarizations.”
Such measurements made using linearly polarized lasers can determine if the fragments’ rotation axis is parallel to the plane of light polarization or perpendicular to it, but linearly polarized light is too symmetrical to distinguish up from down, left from right, or clockwise from counterclockwise. Yet these distinctions are predicted to be the clearest signature of the quantum interference between paths.
In the newly reported work using circularly polarized lasers — where the electric field spirals around the direction of beam propagation like a corkscrew — the Brookhaven team has observed previously invisible patterns that are related to which fragments are rotating in the same direction as the laser light, and which in the opposite orientation.
“These subtleties of orientation are directly related to the phenomenon of quantum interference in photodissociation, and we are the first to measure how the orientation depends on the direction of the fragments’ recoil velocity as these light-sensitive molecules fall apart,” Hall said.
The results are helping Hall and other chemical physicists around the world understand how phase affects chemical reactivity and how manipulation of phases with lasers may be used to control the outcome of chemical reactions.
Source: Brookhaven National Laboratory