Researchers decipher the shape of the sodium/potassium ion pump
Proteins studding the surface of cell membranes are vital to the cell, transmitting signals and maintaining equilibrium by moving charged molecules — ions — from one side to the other. Some of these proteins even use energy by acting as pumps, specialized channels with gates that strictly open one at a time to usher ions in and out in a tightly controlled manner.
The sodium/potassium (Na/K) ion pump is one of the most prevalent: It occurs in every cell in the human body, and is a frequent target of heart-failure medications. But although researchers have a reasonable model of the protein’s outer structure, they’re still working to determine the shape of its inner channel and, therefore, still trying to understand how best to manipulate it.
Two new papers from Rockefeller University professor David Gadsby, head of the Laboratory of Cardiac and Membrane Physiology, go a long way toward describing the shape of the gated channel in the Na/K pump and providing an understanding of how this pump — and others like it — collects ions on one side and releases them on the other.
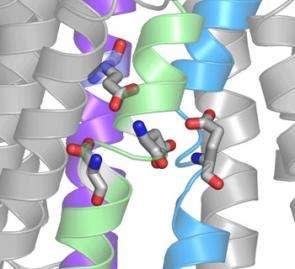
Many of the residues in an ion pump have an electrical charge that works to either draw in or repel approaching ions, allowing only those with the correct charge to flow through to their destination. Using a lethal marine toxin, Gadsby and postdoctoral associates Pablo Artigas and Nicolбs Reyes disabled the gates of the Na/K pump and used an amplifying method called the patch-clamp technique to measure ion flow through individual protein pumps. Once they’d unlocked the channel gates, the researchers attached molecular markers to amino acids within the channel and then sent charged chemicals through it to see whether, and how, interactions between the molecules impacted the ion stream.
In research published in August in the Proceedings of the National Academy of Sciences, Gadsby and Artigas studied two of the protein’s amino acids that react with digoxin, a cardiac drug known to target the Na/K pump. By altering the two residues it binds to and measuring the resulting change in the ion stream, the scientists were able to determine where those residues are located: in a wide opening near the mouth of the channel.
A month later, in a paper in the September 28 issue of Nature, Gadsby and Reyes used the same residue-altering technique to further untangle the shape of the ion pathway. Their results show that two adjacent residues have a vastly different effect on ion flow: Altering one of them slows the current, while altering the other stops it completely. This, Gadsby says, implies that the ion channel is shaped much like a funnel, with a wide outer region that narrows quite suddenly. Deeper in the narrow neck of the funnel is a filter, made of negatively charged residues, that selects for positively charged ions. By altering just a single residue to reverse its charge, the researchers were actually able to switch the selectivity of the filter. “We’d taken this beautiful pump, which had evolved to attract positive sodium and potassium ions and bind them, and completely reversed its preference to negative ions,” he says.
Their findings have implications that go beyond the Na/K pump: Because it’s in the same family as the stomach’s acid-producing pump, “we can argue that the shape of this pathway is going to be conserved,” Gadsby says. If this holds true, he has laid bare a more precise target for future antacid drugs.
Citation:
-- Proceedings of the National Academy of Sciences 103(33): 12613-12618 (August 15, 2006)
-- Nature 443: 470-474 (September 28, 2006)
Source: Rockefeller University